Soaps and detergents are essential cleaning agents widely used in households and industries. They are both surfactants, which means they help reduce the surface tension of water, making it easier to remove dirt, grease, and stains. Despite their similarities, soaps and detergents have some key differences in terms of their chemical composition and how they function in different water conditions.
Soaps
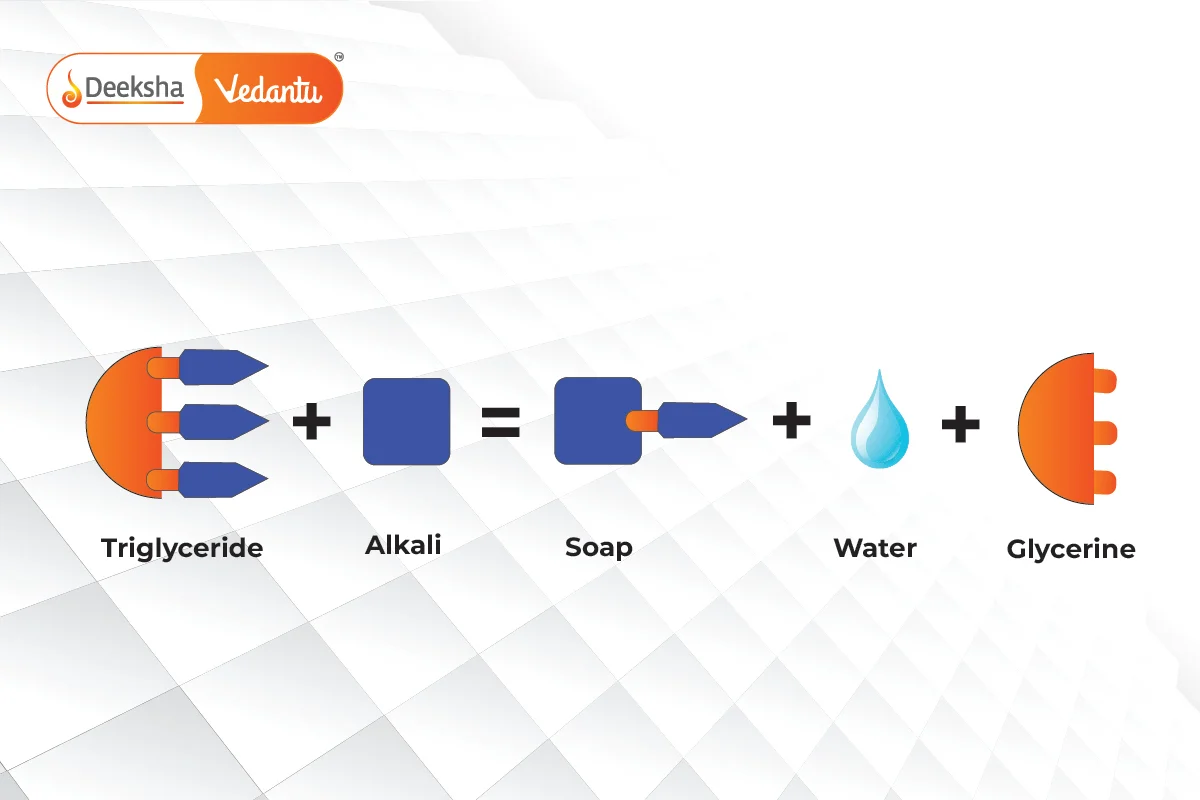
Soaps are the sodium or potassium salts of long-chain fatty acids, typically derived from fats or oils. The process of making soap is known as saponification. During saponification, fats or oils (which are esters of fatty acids and glycerol) are heated with sodium hydroxide () or potassium hydroxide (
) to produce soap and glycerol.
Chemical Reaction – Saponification:
Soaps are amphiphilic molecules, meaning they have both a hydrophobic tail (which repels water and binds to grease and oil) and a hydrophilic head (which attracts water). This dual nature is what makes soaps effective for cleaning.
Micelles:
When soap molecules interact with water and grease, they arrange themselves into spherical structures called micelles. The hydrophobic tails of the soap molecules surround the oil or grease, trapping it inside the micelle, while the hydrophilic heads face outward toward the water. This structure allows the grease or dirt to be rinsed away with water.
Real-life Applications:
- Personal Hygiene: Soaps are used for bathing and handwashing as they effectively remove dirt, oils, and bacteria from the skin.
- Laundry: In areas with soft water, soap is still commonly used for washing clothes as it can remove oils and stains.
Detergents
Detergents are synthetic cleansing agents that are typically derived from petrochemicals. Unlike soaps, detergents are effective even in hard water, which contains high levels of calcium () and magnesium (
) ions. These ions do not react with detergents, allowing them to remain effective without forming scum.
Types of Detergents:
- Anionic Detergents: These detergents have negatively charged hydrophilic heads. Example: Sodium lauryl sulfate, commonly used in shampoos and laundry detergents.
- Cationic Detergents: These detergents have positively charged hydrophilic heads. Example: Quaternary ammonium salts, which are often used in fabric softeners.
- Non-ionic Detergents: These detergents do not have any charge in their hydrophilic part. Example: Polyethylene glycol (PEG)-based detergents, which are used in gentle cleaning products.
How Detergents Work:
Like soaps, detergents also work by forming micelles. The hydrophobic tails of the detergent molecules trap the grease or oil, while the hydrophilic heads interact with water, allowing the trapped dirt to be washed away.
Real-life Applications:
- Laundry Detergents: Detergents are highly effective in cleaning clothes, particularly in areas with hard water, as they do not form scum.
- Dishwashing Liquids: Detergents used for washing dishes contain surfactants that help break down grease and remove food particles.
- Industrial Cleaning: Detergents are used in industries such as food processing and pharmaceuticals for cleaning equipment and surfaces.
Comparison: Soaps vs. Detergents
| Property | Soap | Detergent |
| Source | Derived from natural fats and oils. | Synthesized from petrochemicals. |
| Effectiveness in Hard Water | Less effective, forms scum in hard water. | Effective in hard water, does not form scum. |
| Biodegradability | Biodegradable and environmentally friendly. | Some detergents are non-biodegradable, leading to pollution. |
| Common Uses | Personal hygiene, laundry (in soft water). | Laundry, dishwashing, industrial cleaning. |
Real-life Applications of Soaps and Detergents
Soaps:
- Personal Hygiene: Soaps are used in various forms such as bars and liquids for washing the body and hands.
- Household Cleaning: Soaps can be used to clean floors, dishes, and fabrics, especially in areas with soft water.
Detergents:
- Laundry: Detergents are formulated with surfactants, enzymes, and bleaches to clean clothes, especially in hard water conditions.
- Dishwashing: Liquid detergents are highly effective in removing grease and food particles from utensils and dishes.
- Industrial Use: Detergents are used in industrial cleaning, including machinery and heavy equipment, where stronger cleaning action is required.
How Soaps and Detergents Work
Both soaps and detergents clean by emulsifying grease and oil, making it possible to rinse them away with water. Here’s a simplified process:
- Breaking Surface Tension: Surfactants in soaps and detergents reduce the surface tension of water, allowing it to spread more easily over surfaces.
- Formation of Micelles: The hydrophobic tails of the surfactant molecules surround and trap oil and grease, forming micelles. The hydrophilic heads of the surfactant molecules face the water.
- Removal of Dirt: The micelles are suspended in the water, and when rinsed, they carry the trapped dirt and grease away, leaving the surface clean.
Practice Questions with Answers
Q1: Write the chemical equation for the saponification reaction.
- Answer:
Q2: Explain why detergents are more effective than soaps in hard water.
- Answer: Detergents are more effective in hard water because they do not react with calcium and magnesium ions, allowing them to remain soluble and clean effectively without forming scum.
Q3: What is the environmental impact of using synthetic detergents?
- Answer: Some synthetic detergents are non-biodegradable and can pollute water bodies, leading to environmental damage. Many modern detergents are now designed to be biodegradable to mitigate this impact.
FAQs
Detergents do not react with calcium and magnesium ions in hard water, so they do not form scum. This makes them more effective cleaners in areas with hard water.
A micelle is a spherical structure formed by soap or detergent molecules, with hydrophobic tails trapping grease and hydrophilic heads interacting with water. This allows dirt to be washed away easily.
In hard water, calcium and magnesium ions react with soap molecules to form an insoluble precipitate called scum, which reduces the soap’s effectiveness.
Related Topics
- Understanding the Chemical Properties of Acids and Bases
- Types of Chemical Reactions
- 118 Elements – Their Symbols and Atomic Number
- Differential Extraction Chromatography
- Protein Structure And Levels of Protein
- Hybridization
- Isomerism
- Classification of Carbohydrates and its Structure
- Reactivity Series
- How Strong Are Acid Or Base Solutions?
- Metals and Non-Metals
- Bonding In Carbon – The Covalent Bond
- Occurrence of Metals
- Periodicity of Valence or Oxidation States of Elements
- Some Important Carbon Compounds – Ethanol And Ethanoic Acid










Get Social