What Are Semiconductors?
Semiconductors are materials characterized by their conductivity, which falls between that of conductors (like metals) and non-conductors or insulators (such as ceramics). These materials can be elemental, like silicon and germanium, or compound-based, such as gallium arsenide. Semiconductors form the foundation of modern electronics, influencing both theory and practical applications in physics.
Electrons and Holes: Charge Carriers in Semiconductors
In the realm of semiconductors, current flow is facilitated by two types of charge carriers: electrons and holes. Electrons are negatively charged particles, while holes, which represent the absence of an electron in the valence band, behave as positively charged particles. The unique interaction between these carriers allows semiconductors to conduct electricity under certain conditions.
Mobility of Electrons and Holes
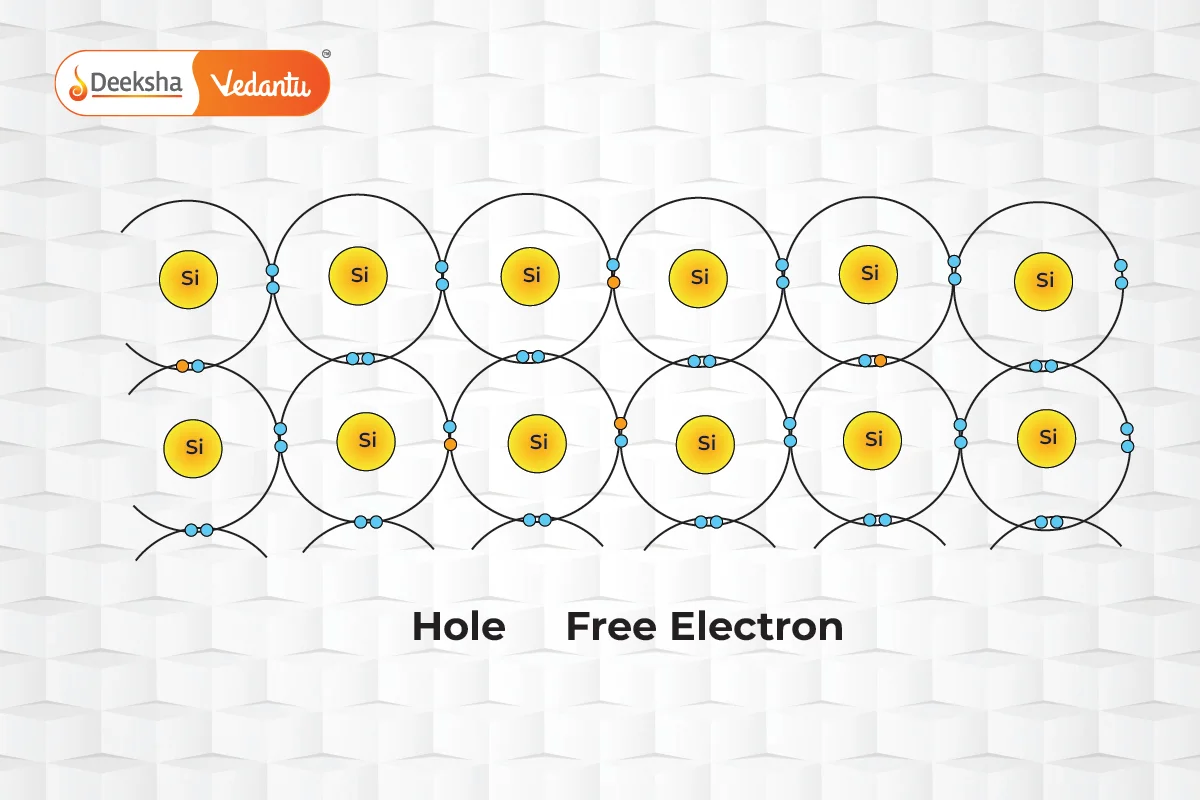
The ability of charge carriers to move through the semiconductor material defines their mobility. Electrons, which travel in the conduction band, generally exhibit higher mobility than holes, which navigate through the more restrictive valence band. Factors influencing mobility include the effective mass of the particles and the frequency of scattering events. For example, in intrinsic silicon at room temperature, electron mobility is approximately 1500 cm²/(V·s), significantly higher than the 475 cm²/(V·s) mobility for holes.
Band Theory of Semiconductors
The band theory offers a quantum-level explanation of how electrons behave in solids. Electrons in an atom occupy specific energy levels. In a solid’s structure, these levels blend into bands:
- Valence Band: The highest energy band filled with electrons.
- Conduction Band: A higher energy band that is typically vacant and can accept electrons excited from the valence band.
- Band Gap: The energy difference between the conduction band and the valence band.
Types of Semiconductors
1. Intrinsic Semiconductors:
- Pure semiconductors without any significant impurities.
- Electron-hole pair generation is solely due to thermal energy.
2. Extrinsic Semiconductors:
- Doped with impurities to enhance conductivity.
- Classified into n-type (extra electrons) and p-type (extra holes) based on the type of dopant used.
Properties of Semiconductors
Semiconductors exhibit unique properties that make them essential in various applications:
- Variable Conductivity: Under different conditions, semiconductors can act as conductors or insulators.
- Negative Temperature Coefficient: Their resistance decreases as temperature increases.
- Controlled by Doping: The electrical properties can be precisely controlled by adding impurities.
Applications of Semiconductors
Semiconductors have revolutionized the field of electronics. They are integral in:
- Computing: CPUs and memory devices.
- Telecommunications: Transistors, diodes, and photodetectors.
- Consumer Electronics: Everything from smartphones to home appliances.
- Photovoltaics: Solar cells convert light into electricity.
FAQs
Silicon and germanium are the most widely used elemental semiconductors, while compounds like gallium arsenide are crucial for specific applications like LEDs and lasers.
Yes, intrinsic semiconductors can conduct electricity but much less efficiently than extrinsic semiconductors, which are enhanced by doping.
N-type semiconductors have extra electrons as charge carriers, making them negatively charged. P-type semiconductors have holes as charge carriers, giving them a positive charge.
As temperature increases, more electrons gain enough energy to jump from the valence band to the conduction band, decreasing the material’s overall resistance.
A semiconductor is defined by its band gap which is small enough to allow the excitation of electrons from the valence band to the conduction band under normal conditions.










Get Social