Rutherford’s atomic model, also known as the nuclear atom or planetary model, was proposed by Ernest Rutherford in 1911. This model describes the atom as a small, dense, positively charged nucleus at the center, with negatively charged electrons orbiting around it, similar to how planets orbit the sun.
Rutherford’s Alpha Scattering Experiment Explanation
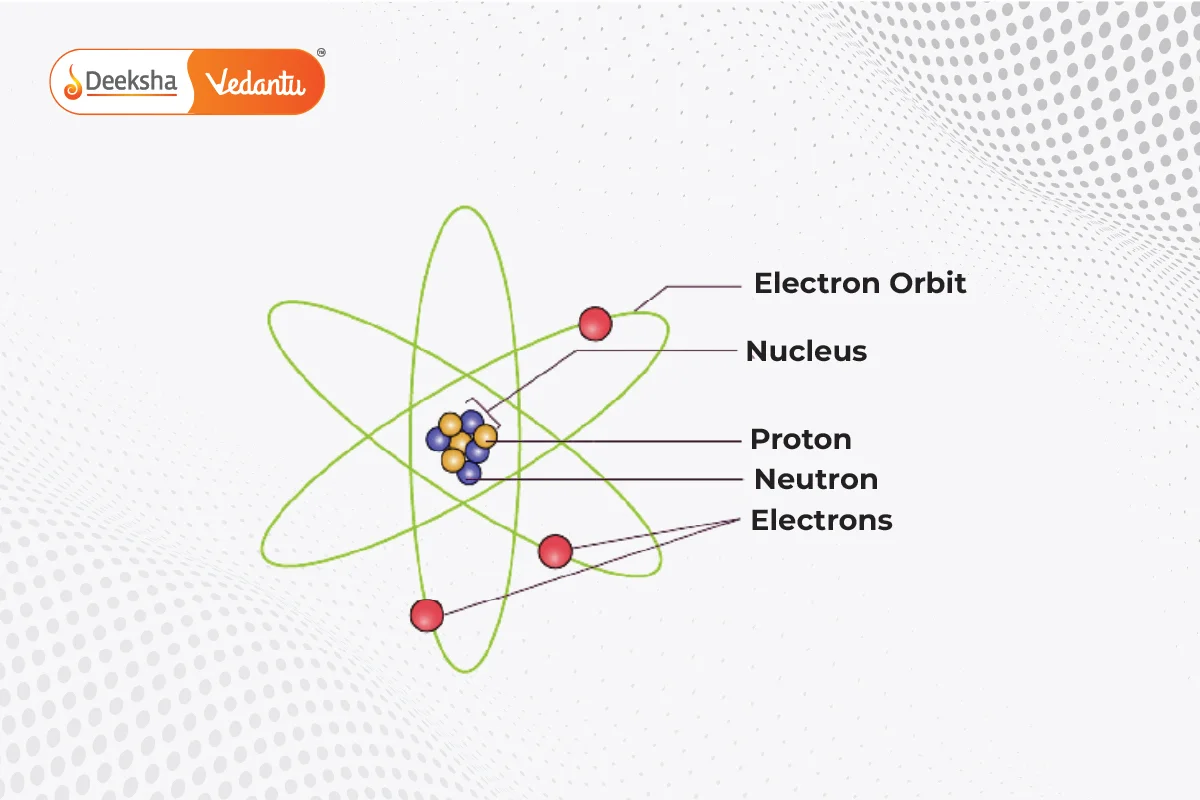
Key Experiments: The Gold Foil Experiment
In the gold foil experiment, Rutherford and his team bombarded a thin sheet of gold foil with alpha particles (positively charged particles). They observed how these particles were scattered after hitting the foil.
Setup:
- A radioactive source emitted alpha particles, which were directed towards the gold foil through a slit in a lead screen.
- A zinc sulfide-coated screen detected the particles by producing flashes of light when hit by alpha particles, visible through a microscope.
Observations:
- Most alpha particles passed through the foil without deflection, suggesting that atoms are mostly empty space.
- Some particles experienced slight deflections, indicating the presence of a positive charge within the atom.
- A few particles were deflected at large angles or bounced back, implying that the positive charge is concentrated in a small, dense region.
Conclusions from the Experiment
Rutherford concluded that:
- The atom has a small, dense nucleus containing most of its mass and positive charge.
- Electrons orbit this nucleus at a distance, occupying most of the atom’s volume.
- The number of electrons equals the number of protons in the nucleus, making the atom electrically neutral.
Features of Rutherford’s Atomic Model
- The nucleus is very small and dense, containing all the positive charge due to protons.
- Different elements have different numbers of protons, giving them different positive charges.
- Electrons, which are negatively charged, orbit the nucleus at high speeds.
- Most of the atom is empty space.
Drawbacks of Rutherford’s Atomic Model
- Stability of Atoms: The model couldn’t explain why atoms are stable. According to classical physics, orbiting electrons should lose energy and spiral into the nucleus, causing the atom to collapse.
- Hydrogen Spectrum: The model also couldn’t explain the existence of specific spectral lines in the hydrogen atom’s emission spectrum.
Rutherford’s model was a significant step forward in understanding atomic structure, but it was later refined by Niels Bohr, who introduced concepts from quantum theory to address its limitations.
FAQs
The hydrogen spectrum problem refers to the fact that Rutherford’s model could not explain why hydrogen atoms emit light at specific wavelengths, forming a series of discrete spectral lines. This was later explained by Bohr’s model using quantum theory.
According to classical physics, orbiting electrons should continuously emit energy and lose speed, eventually collapsing into the nucleus. This would make atoms unstable, which contradicts the observed stability of matter.
Rutherford’s model laid the groundwork for future atomic theories by introducing the concept of a nucleus. It was later refined by Niels Bohr, who incorporated quantum theory to explain the stability of atoms and the hydrogen spectrum.
The nucleus is important because it contains almost all the mass of the atom and the positive charge, which influences the behavior and arrangement of the electrons.
Rutherford developed his model based on the gold foil experiment conducted in 1909. This experiment involves bombarding a thin gold foil with alpha particles and observing their scattering patterns.
Rutherford’s atomic model, also known as the nuclear atom or planetary model, describes the atom as having a small, dense nucleus at the center, containing all the positive charge, with electrons orbiting around it, similar to how planets orbit the sun.
Related Topics
- More About Salts
- Bonding In Carbon – The Covalent Bond
- Suspension
- 118 Elements – Their Symbols and Atomic Number
- Metals and Non-Metals
- First 20 Elements of the Periodic Table
- Soil Pollution
- Natural Resources
- Chemical Properties Of Carbon Compounds
- Differential Extraction Chromatography
- Reactivity Series
- Some Important Carbon Compounds – Ethanol And Ethanoic Acid
- Corrosion
- Chemical Properties Of Metals
- What Do All Acids And All Bases Have In Common?










Get Social