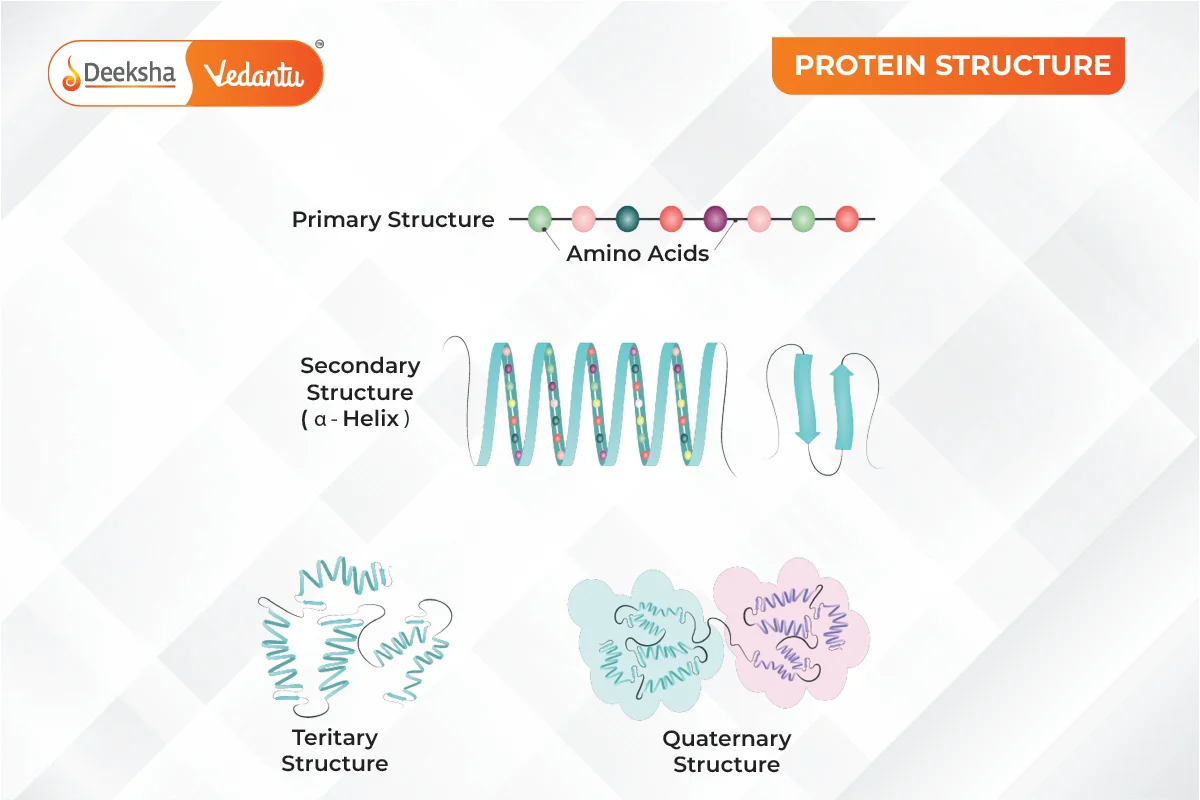
You might not realize it, but protein is the most abundant substance in your body after water. Every cell in your body contains protein. Proteins have a unique 3D shape, which allows them to perform various functions. Proteins are made up of amino acids linked by peptide bonds. There are four levels of protein structure: primary, secondary, tertiary, and quaternary. The primary structure is the sequence of amino acids. The secondary structure involves the angles of peptide bonds, while the tertiary structure is about the folding of protein chains. This article explains protein structures in a simple way.
Protein Structure Definition
Proteins are biological polymers made of amino acids, the building blocks of proteins. Proteins have a chain-like structure, where amino acids are the primary components. The structure of proteins is more complex than that of small molecules, with four levels: primary, secondary, tertiary, and quaternary.
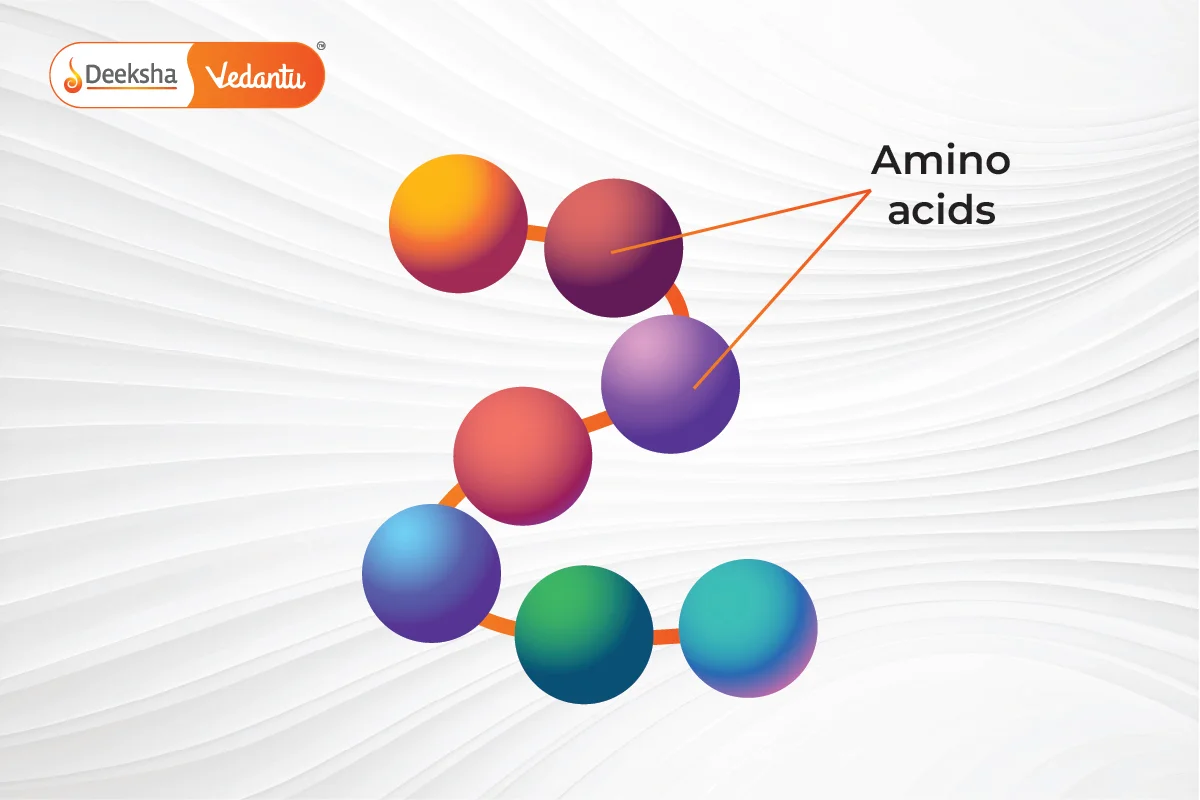
Amino acids link together with peptide bonds. When several peptide bonds form, they create a polypeptide chain. One or more of these chains can twist or fold to form a protein.
The size of a protein varies based on the number of polypeptide molecules it contains. For example, insulin is one of the smallest proteins, while titin is the largest, with 34,350 amino acids.
Classification of Proteins
Proteins can be classified as fibrous or globular based on their shape. Fibrous proteins have polypeptide chains running parallel and bonded by hydrogen and disulfide bonds, creating a fiber-like structure. Globular proteins have coiled chains that form a spherical shape.
Levels of Protein Structure
1. Primary Structure of Protein
The primary structure of a protein is the exact sequence of amino acids in its chain. This sequence is crucial because it determines how the protein will fold and function. The arrangement of amino acids in a specific order is characteristic of each protein. Any change in this sequence can alter the entire protein’s function. This sequence is encoded in the DNA. If there’s a mutation in the DNA, the protein’s function might be affected. The primary structure is maintained by covalent peptide bonds between amino acids. Many genetic disorders, like cystic fibrosis and sickle cell anemia, result from mutations that change the primary structure, affecting the protein’s other structures.
2. Secondary Structure of Protein
The secondary structure refers to the local folding of the polypeptide chain due to interactions between the backbone atoms. Proteins do not remain as simple chains; they fold due to interactions between the amine and carboxyl groups of the peptide link. This structure includes shapes like α-helix and β-pleated sheet, formed by hydrogen bonds between -CO and -NH groups of the peptide bond.
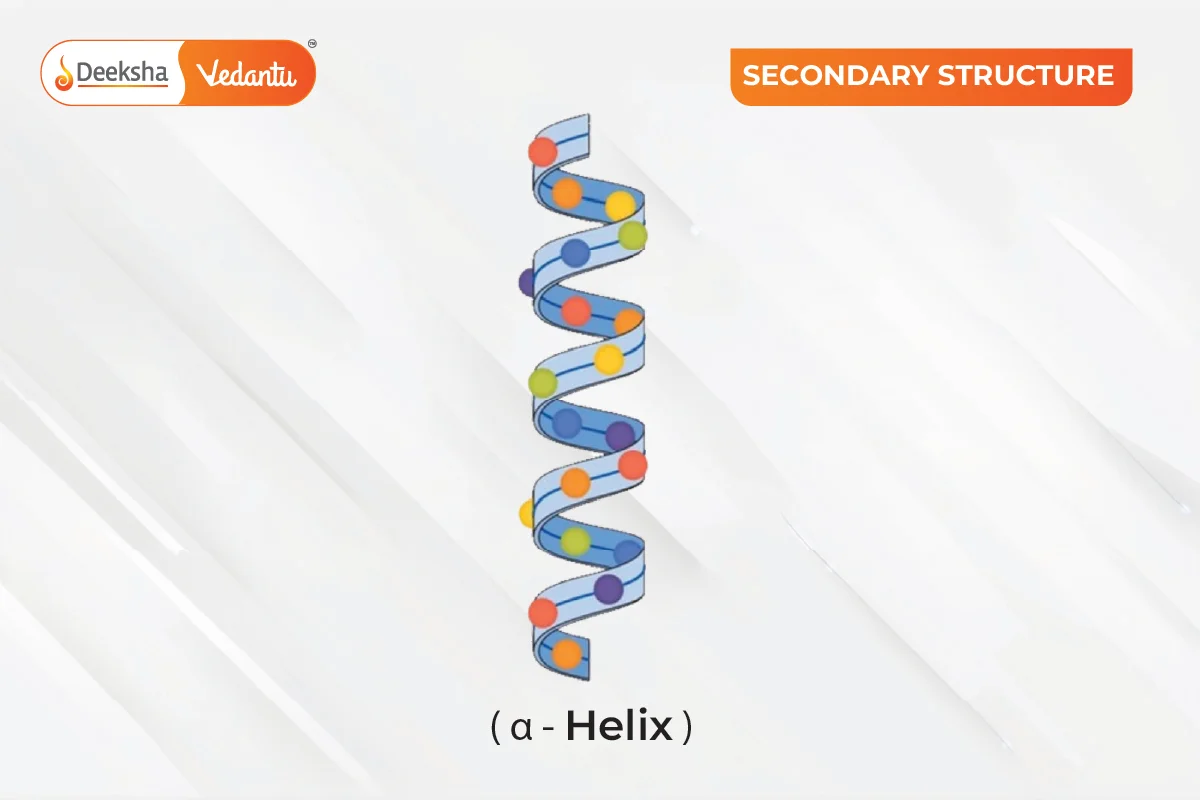
α-Helix: A common structure where the polypeptide chain twists into a right-handed spiral, with hydrogen bonds forming between the -NH group of one amino acid and the -CO group of another.
β-Pleated Sheet: Polypeptide chains are stretched out and laid side by side, held together by hydrogen bonds, resembling the pleated folds of drapery.
3. Tertiary Structure of Protein
The tertiary structure arises from further folding of the secondary structure into a unique three-dimensional shape. This structure is stabilized by hydrogen bonds, electrostatic forces, disulfide linkages, and van der Waals forces. Tertiary structures can form two major shapes: fibrous and globular. The folding determines the protein’s overall shape and function.

4. Quaternary Structure of Protein
The quaternary structure involves the spatial arrangement of multiple polypeptide chains or subunits. These subunits interact to form a functional protein. The arrangement of these subunits with respect to each other is what constitutes the quaternary structure.

Rules of Protein Structure
- The function of a protein is determined by its type.
- A protein’s shape is dictated by its primary structure (amino acid sequence).
- The amino acid sequence is encoded by the DNA gene sequence.
- The unique sequence of amino acids drives the protein to fold into its specific and active three-dimensional shape (tertiary structure).
Summary
Proteins are complex molecules made up of amino acids linked by peptide bonds, forming primary, secondary, tertiary, and quaternary structures. These structures determine the protein’s function and stability. Changes in the amino acid sequence can lead to significant alterations in protein function, underlying many genetic disorders. Understanding these levels of structure is crucial in the study of protein function and biology.
FAQs
The tertiary structure is the overall three-dimensional folding of a polypeptide chain, stabilized by various interactions like hydrogen bonds, electrostatic forces, disulfide linkages, and van der Waals forces.
Mutations in the DNA can change the amino acid sequence in the protein’s primary structure, potentially altering its folding and function, leading to genetic disorders.
The quaternary structure refers to the spatial arrangement of multiple polypeptide chains or subunits in a protein, resulting in a functional protein complex.
The tertiary structure is stabilized by hydrogen bonds, electrostatic forces, disulfide linkages, and van der Waals forces, which maintain the protein’s unique shape.
An α-helix is a right-handed spiral formed by hydrogen bonds between the -NH group of one amino acid and the -CO group of another. A β-pleated sheet consists of polypeptide chains laid side by side, bonded by hydrogen bonds, creating a sheet-like structure.
The secondary structures of proteins are local folded shapes within a polypeptide chain, such as α-helix and β-pleated sheet, stabilized by hydrogen bonds between the backbone atoms.
The amino acid sequence is crucial because it dictates the protein’s final three-dimensional shape, which is essential for its specific function. Any change in this sequence can alter the protein’s function.
The primary structure of a protein is the exact sequence of amino acids in its polypeptide chain. This sequence determines how the protein will fold and function.
Related Topics
- Physical Properties Of Metals And Non-Metals
- Modern Periodic Table
- Versatile Nature Of Carbon
- Rutherford’s Model of Atoms and its Limitations
- How Do Metals and Non-Metals React?
- Bonding In Carbon – The Covalent Bond
- Electronic Configuration of First 30 Elements
- Chemical Properties Of Metals
- Isomerism
- Periodicity of Valence or Oxidation States of Elements
- How Strong Are Acid Or Base Solutions?
- First 20 Elements of the Periodic Table
- Corrosion
- Reactivity Series
- Acids, Bases, and Salts






Get Social