Introduction to Periodicity of Valence States
Valency is a measure of an element’s ability to combine with other elements. It is defined by the number of electrons an atom needs to gain or lose to achieve a stable electron configuration.
What is an Oxidation State?
The oxidation state of an atom indicates the number of electrons gained or lost by it.
Valency and oxidation states are key properties of elements, determined by their electron configurations. Valency is the number of electrons an atom must lose or gain to achieve stability, while the oxidation state is the charge of an atom due to electron loss or gain.
Valency and Oxidation State
Valence electrons are those in an atom’s outermost shell and define its valency. For elements in the s-block and p-block of the periodic table, valency is often calculated as either the number of valence electrons or eight minus that number.
For d-block and f-block elements, valency depends on both valence electrons and electrons in d and f orbitals, with common valencies being 2 and 3.
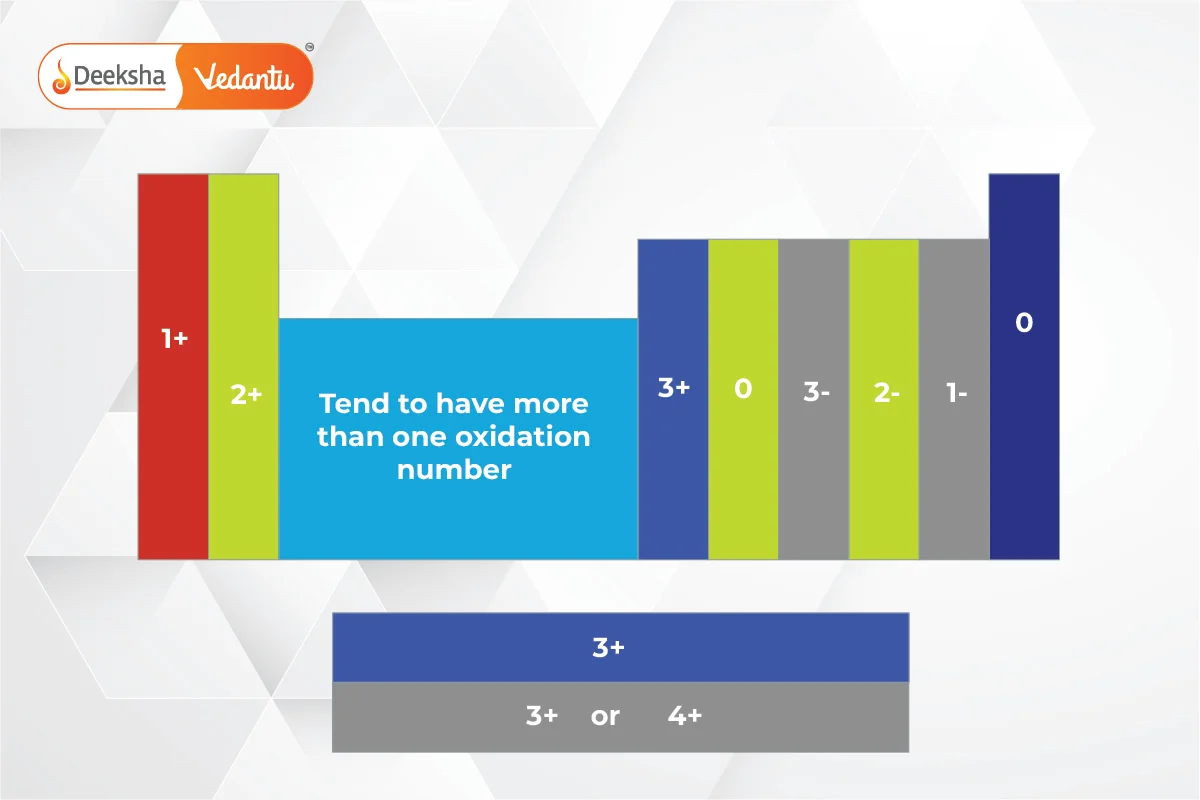
Valency of the First 30 Elements in the Periodic Table
Here’s a table listing the valency of the first 30 elements based on their electron configurations:
| Element | Symbol | Atomic Number | Valency |
| Hydrogen | H | 1 | 1 |
| Helium | He | 2 | 0 |
| Lithium | Li | 3 | 1 |
| Beryllium | Be | 4 | 2 |
| Boron | B | 5 | 3 |
| Carbon | C | 6 | 4 |
| Nitrogen | N | 7 | 3 |
| Oxygen | O | 8 | 2 |
| Fluorine | F | 9 | 1 |
| Neon | Ne | 10 | 0 |
| Sodium | Na | 11 | 1 |
| Magnesium | Mg | 12 | 2 |
| Aluminum | Al | 13 | 3 |
| Silicon | Si | 14 | 4 |
| Phosphorus | P | 15 | 3 |
| Sulfur | S | 16 | 2 |
| Chlorine | Cl | 17 | 1 |
| Argon | Ar | 18 | 0 |
| Potassium | K | 19 | 1 |
| Calcium | Ca | 20 | 2 |
| Scandium | Sc | 21 | 3 |
| Titanium | Ti | 22 | 4 |
| Vanadium | V | 23 | 5,4 |
| Chromium | Cr | 24 | 2 |
| Manganese | Mn | 25 | 7,4,2 |
| Iron | Fe | 26 | 2,3 |
| Cobalt | Co | 27 | 3,2 |
| Nickel | Ni | 28 | 2 |
| Copper | Cu | 29 | 2,1 |
| Zinc | Zn | 30 | 2 |
This table summarizes the valency of the first 30 elements, showing how many electrons each element can gain, lose, or share to form a stable electron configuration.
Trends in Oxidation States
Across a Period: Moving left to right, the number of valence electrons increases from 1 to 8. Valency increases from 1 to 4 and then decreases to zero when combined with hydrogen or oxygen. For example, in Na2O and F2O, F is more electronegative than O in F2O, giving F a -1 oxidation state and O a +2. In Na2O, O is more electronegative, giving O a -2 oxidation state and Na a +1.
Within a Group: The number of valence electrons remains constant down a group, so elements in the same group have the same valency.
Guidelines for Assigning Oxidation States
- Elements in their natural form (O2, H2, etc.) have an oxidation state of zero.
- Oxygen typically has an oxidation state of -2, except in peroxides where it is -1.
- Hydrogen is usually +1, but -1 in metal hydrides (e.g., NaH).
- Halogens are generally -1 unless combined with oxygen or each other.
- Alkali metals (Na, K, etc.) have an oxidation state of +1.
- Alkaline earth metals (Mg, Ca, etc.) have an oxidation state of +2.
Finding Valency of Elements
Valency is determined by the number of electrons in the outer shell. One method to find valency is by consulting the periodic table, where elements in the same group typically have the same valency. For instance, elements in group 8 have a valency of 8, indicating high stability.
By understanding these basic principles, the periodic trends in valency and oxidation states of elements can be easily comprehended.
FAQs
Elements have different oxidation states due to their ability to lose or gain different numbers of electrons. This variability depends on the element’s electron configuration and its position in the periodic table.
Yes, many elements can have multiple oxidation states. Transition metals, in particular, often exhibit a variety of oxidation states due to their complex electron configurations.
Within a group, the number of valence electrons remains the same, so elements in the same group typically exhibit similar valency and oxidation states.
As you move from left to right across a period, the number of valence electrons increases from 1 to 8. The oxidation state can vary, usually increasing in a similar pattern until reaching group 14, then decreasing.
Valency is a specific case of oxidation state where the atom’s combining capacity is considered without assigning charges. Oxidation state, on the other hand, always involves the effective charge due to electron gain or loss.
For s-block and p-block elements, valency is typically the number of valence electrons or eight minus the number of valence electrons. For d-block and f-block elements, valency includes electrons in both valence and d or f orbitals.
The oxidation state of an atom indicates the number of electrons an atom has gained or lost. It represents the effective charge of an atom in a compound due to the transfer of electrons.
Valency is the measure of an element’s ability to combine with other elements. It represents the number of electrons an atom needs to gain, lose, or share to achieve a stable electron configuration.
Related Topics
- Occurrence of Metals
- Some Important Carbon Compounds – Ethanol And Ethanoic Acid
- Understanding the Chemical Properties of Acids and Bases
- How Do Metals and Non-Metals React?
- Metals and Non-Metals
- Soaps And Detergents
- Types of Chemical Reactions
- How Strong Are Acid Or Base Solutions?
- Electronic Configuration of First 30 Elements
- What Do All Acids And All Bases Have In Common?
- Acids, Bases, and Salts
- Hybridization
- Chemical Formula
- Isomerism
- Physical Properties Of Metals And Non-Metals











Get Social