What is the Modern Periodic Table?
The Modern Periodic Table is an arrangement of elements in order of their increasing atomic number. The atomic number of an element refers to the number of protons present in the nucleus of an atom. This modern arrangement was proposed by Henry Moseley in 1913, and it resolved many of the inconsistencies found in Mendeleev’s Periodic Table, which was based on atomic mass.
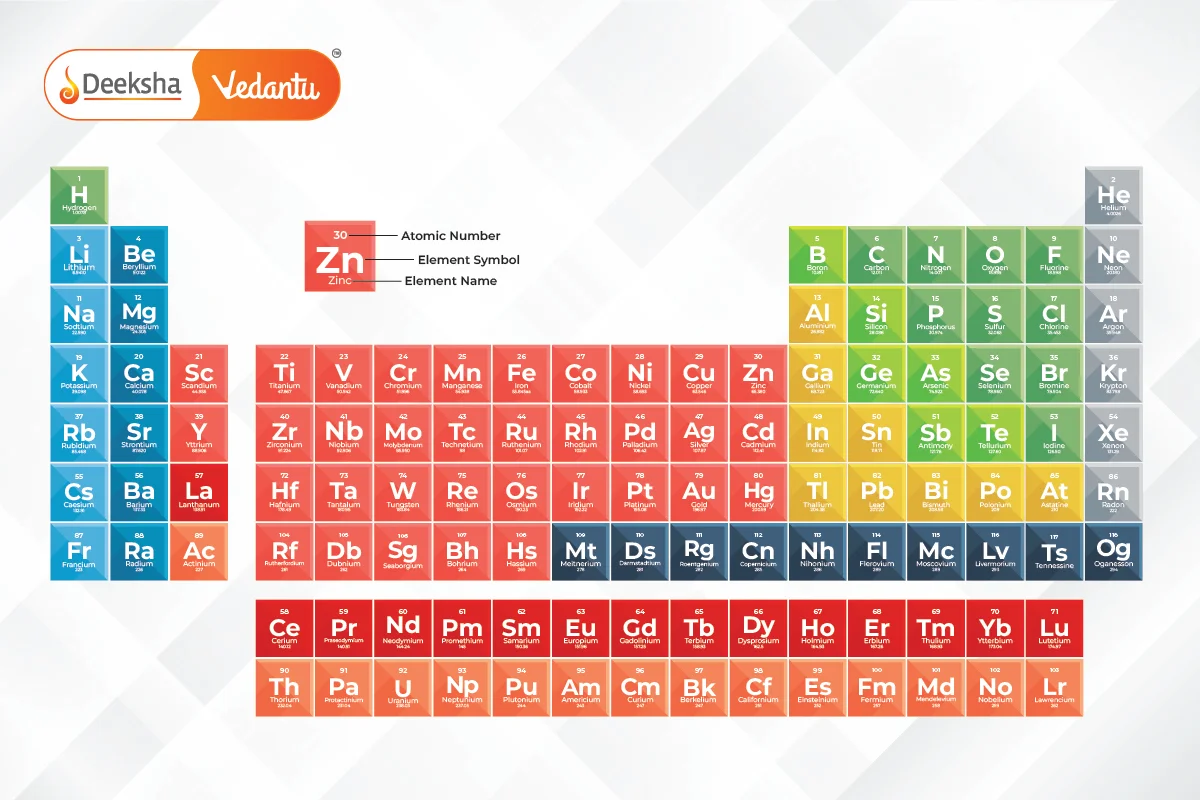
Key Characteristics:
- The table organizes elements into rows (called periods) and columns (called groups).
- Elements in the same group have similar chemical properties because they have the same number of valence electrons.
- The properties of elements exhibit a repeating, or periodic, pattern when arranged by atomic number. This phenomenon is called periodicity.
History and Development of the Modern Periodic Table
The development of the modern periodic table has a long history of contributions from several scientists:
- Dmitri Mendeleev (1869):
- Mendeleev arranged elements by atomic mass and noticed that their chemical properties repeated periodically. He even predicted the existence and properties of elements that had not yet been discovered.
- Limitations: Mendeleev’s table left gaps for undiscovered elements and could not explain why certain elements (like iodine and tellurium) were out of order when arranged by atomic mass.
- Henry Moseley (1913):
- Moseley rearranged the elements based on their atomic number (number of protons) instead of atomic mass.
- Moseley’s discovery of the atomic number as the organizing principle resolved discrepancies in Mendeleev’s table and led to the modern periodic law.
Periodic Law:
“The physical and chemical properties of elements are periodic functions of their atomic numbers.”
This means that when elements are arranged by increasing atomic number, their properties repeat in a predictable pattern.
Structure of the Modern Periodic Table
The modern periodic table is arranged into groups and periods. The placement of an element in the table gives insight into its chemical properties and electron configuration.
- Groups:
- There are 18 groups (vertical columns) in the modern periodic table.
- Elements in the same group have the same number of valence electrons, which gives them similar chemical properties. For example, all elements in Group 1 (alkali metals) have one valence electron and are highly reactive.
- Group 1: Alkali metals (e.g., sodium, potassium)
- Group 17: Halogens (e.g., chlorine, fluorine)
- Group 18: Noble gases (e.g., helium, neon)
- Periods:
- There are 7 periods (horizontal rows).
- The number of electron shells increases as you move down the table. For example, elements in the second period have two electron shells, while elements in the third period have three.
- Blocks: The modern periodic table is divided into four blocks based on the electron configuration of the elements:
- s-block: Groups 1 and 2 (alkali and alkaline earth metals).
- p-block: Groups 13 to 18 (contains metals, metalloids, and non-metals).
- d-block: Transition metals (Groups 3 to 12).
- f-block: Lanthanides and actinides (placed below the main table).
Trends in the Modern Periodic Table
Several trends of periodic properties change predictably across periods and down groups in the modern periodic table. These trends help predict the behavior of elements in chemical reactions.
- Atomic Radius:
- Across a period: Atomic radius decreases from left to right due to increasing nuclear charge, which pulls electrons closer to the nucleus.
- Down a group: Atomic radius increases as additional electron shells are added, making the atom larger.
- Ionization Energy:
- Definition: The energy required to remove an electron from an atom.
- Across a period: Ionization energy increases because the nucleus holds onto its electrons more tightly as the atomic number increases.
- Down a group: Ionization energy decreases because electrons are farther from the nucleus and are easier to remove.
- Electron Affinity:
- Definition: The energy released when an atom gains an electron.
- Across a period: Electron affinity increases as atoms more readily accept electrons to complete their outer shell.
- Down a group: Electron affinity decreases because the added electron is farther from the nucleus, making it less attracted to the atom.
- Electronegativity:
- Definition: The ability of an atom to attract electrons in a chemical bond.
- Across a period: Electronegativity increases as the nucleus has a stronger pull on shared electrons.
- Down a group: Electronegativity decreases because the outer electrons are farther from the nucleus.
- Metallic and Non-Metallic Character:
- Across a period: Metallic character decreases, and non-metallic character increases. For example, sodium (a metal) is on the left, while chlorine (a non-metal) is on the right.
- Down a group: Metallic character increases as atoms more readily lose electrons to form positive ions.
Significance of the Modern Periodic Table
The modern periodic table is an invaluable tool in understanding the chemical behavior of elements and predicting their reactions:
- Predicting Element Properties:
- The position of an element in the table helps predict its chemical reactivity, ionization energy, and atomic radius. For example, elements in Group 1 (alkali metals) are highly reactive and form similar compounds.
- Grouping of Elements:
- Elements with similar properties are grouped together, making it easier for chemists to study trends and behaviors across the table. For example, Group 18 elements are noble gases and are all inert due to their complete electron shells.
- Discovery of New Elements:
- The periodic table has guided the discovery of new elements. For instance, gaps left in Mendeleev’s periodic table were later filled with newly discovered elements like gallium and germanium, which matched his predictions.
- Chemical Reactions:
- Understanding an element’s position in the periodic table allows chemists to predict its behavior in chemical reactions. For instance, elements in Group 17 (halogens) react with metals to form salts, and elements in Group 1 react with water to form hydroxides.
FAQs
Noble gases have a full valence shell of electrons, which makes them highly stable and unreactive compared to other elements.
Rare earth elements mostly comprise the lanthanide series, which are key components in various electronic devices and are known for their magnetic and luminescent properties.
While Mendeleev’s table was organized by increasing atomic mass, the modern table is organized by increasing atomic number, which resolves many of the inconsistencies in the earlier arrangements.
Moseley’s discovery established the atomic number as the basis for organizing the periodic table, leading to a clearer and more accurate understanding of element properties and their relationships.
The modern periodic table helps predict the chemical behavior of elements, organize elements with similar properties, and guide the discovery of new elements. It is a critical tool for chemists.
As you move across a period, the number of protons increases, which increases the nuclear charge. This pulls the electrons closer to the nucleus, reducing the atomic radius.
Periods are horizontal rows, and groups are vertical columns. Elements in the same period have the same number of electron shells, while elements in the same group have the same number of valence electrons.
The periodic law states that the properties of elements are a periodic function of their atomic numbers. This means that elements show recurring patterns in their properties when arranged by atomic number.
Elements are arranged in increasing order of their atomic number (number of protons). This arrangement leads to periodic trends in properties such as atomic radius, ionization energy, and electronegativity.
Related Topics
- Physical Properties Of Metals And Non-Metals
- Suspension
- Understanding the Chemical Properties of Acids and Bases
- Types of Chemical Reactions
- How Do Metals and Non-Metals React?
- Natural Resources
- What Do All Acids And All Bases Have In Common?
- Versatile Nature Of Carbon
- Hybridization
- Acids and Bases
- Isomerism
- Soil Pollution
- Rutherford’s Model of Atoms and its Limitations
- Some Important Carbon Compounds – Ethanol And Ethanoic Acid
- First 20 Elements of the Periodic Table











Get Social