Introduction
Acids, bases, and salts are fundamental chemical compounds that play an essential role in both nature and our daily lives. From the sour taste of citrus fruits to the bitter feel of soap, these substances are all around us, shaping the world in which we live. Acids, which produce hydrogen ions in solution, are known for their sour taste and reactivity with metals, while bases, which release hydroxide ions, are characterized by their bitter taste and slippery feel. When acids and bases interact, they undergo a neutralization reaction, forming salts—an integral part of everyday life, whether in the form of common table salt or the compounds used in antacids. Understanding the chemical properties, reactions, and real-world applications of acids, bases, and salts not only enriches our knowledge of chemistry but also helps us appreciate how these compounds influence our health, the environment, and various industries.
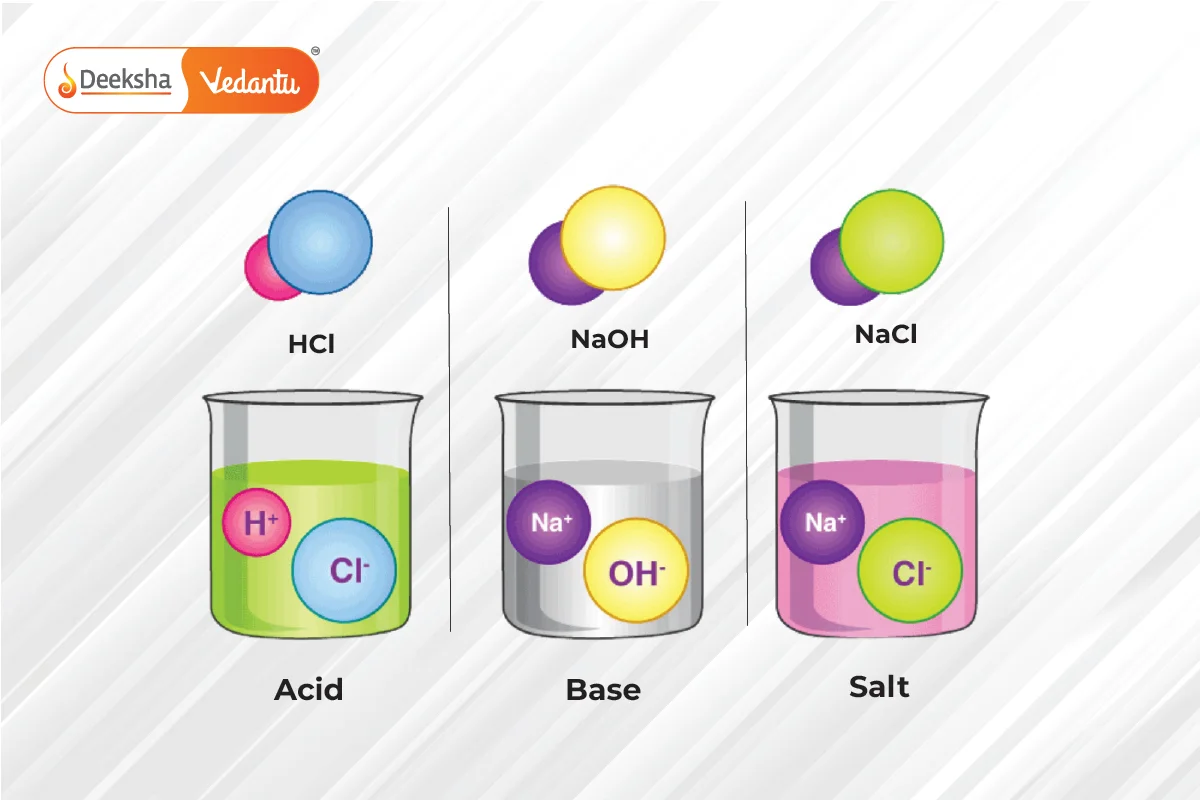
Acids
- Definition: Acids are substances that produce hydrogen ions (
) when dissolved in water.
- Properties:
- Sour taste (think of lemon or vinegar).
- Corrosive to metals and skin.
- Turns blue litmus paper red.
- Conducts electricity in aqueous solution.

- Examples:
- Hydrochloric acid
: Found in the stomach, aids digestion.
- Sulphuric acid
: Used in car batteries.
- Acetic acid
: Found in vinegar.
- Hydrochloric acid
- Uses:
- Sulphuric acid: Used in the manufacture of fertilizers, detergents, and car batteries.
- Acetic acid: Used in food preservation as vinegar.
Bases
- Definition: Bases are substances that produce hydroxide ions (
) when dissolved in water.
- Properties:
- Bitter taste (think of baking soda).
- Soapy to touch.
- Turns red litmus paper blue.
- Conducts electricity in aqueous solution.

- Examples:
- Sodium hydroxide
: Used in making soap.
- Calcium hydroxide
: Used in limewater.
- Sodium hydroxide
- Uses:
- Sodium hydroxide: Used in soap making and the textile industry.
- Calcium hydroxide: Used to neutralize acidic soils in agriculture.
Salts
- Definition: Salts are ionic compounds formed by the neutralization of an acid and a base.
- Examples:
- Sodium chloride
: Common table salt.
- Calcium carbonate
: Found in chalk and used in antacids.
- Sodium chloride
- Uses:
- Sodium chloride: Essential for human health, used in cooking.
- Calcium carbonate: Used in antacids and construction.
Properties of Acids and Bases
| Property | Acids | Bases |
| Taste | Sour (e.g., lemon juice) | Bitter (e.g., soap) |
| Texture | No characteristic texture | Slippery (soapy touch) |
| Litmus Test | Turns blue litmus paper red | Turns red litmus paper blue |
| Electrical Conductivity | Conducts in solution | Conducts in solution |
Chemical Properties:
- Reaction with Metals:
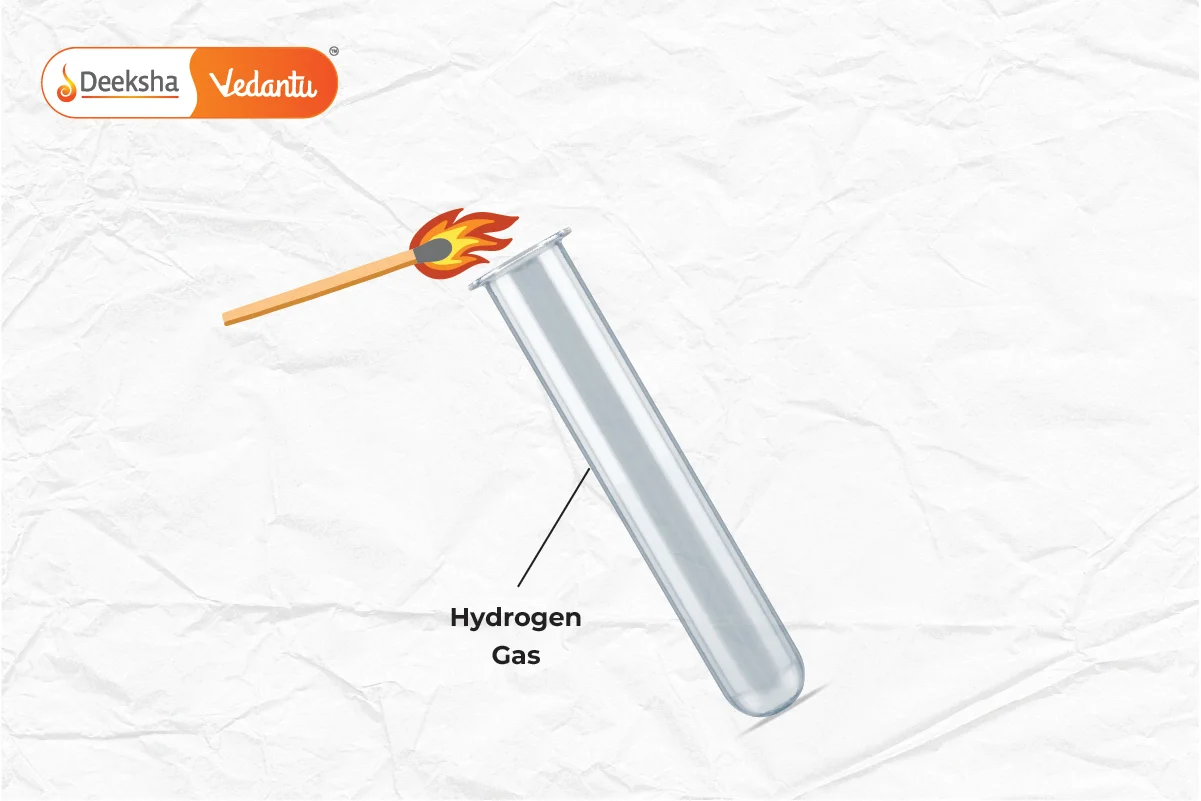
- Acids: React with active metals like zinc and magnesium to release hydrogen gas.
- Example:
- Test for Hydrogen: When hydrogen gas is passed near a burning splint, it burns with a ‘pop’ sound.
- Example:
- Acids: React with active metals like zinc and magnesium to release hydrogen gas.
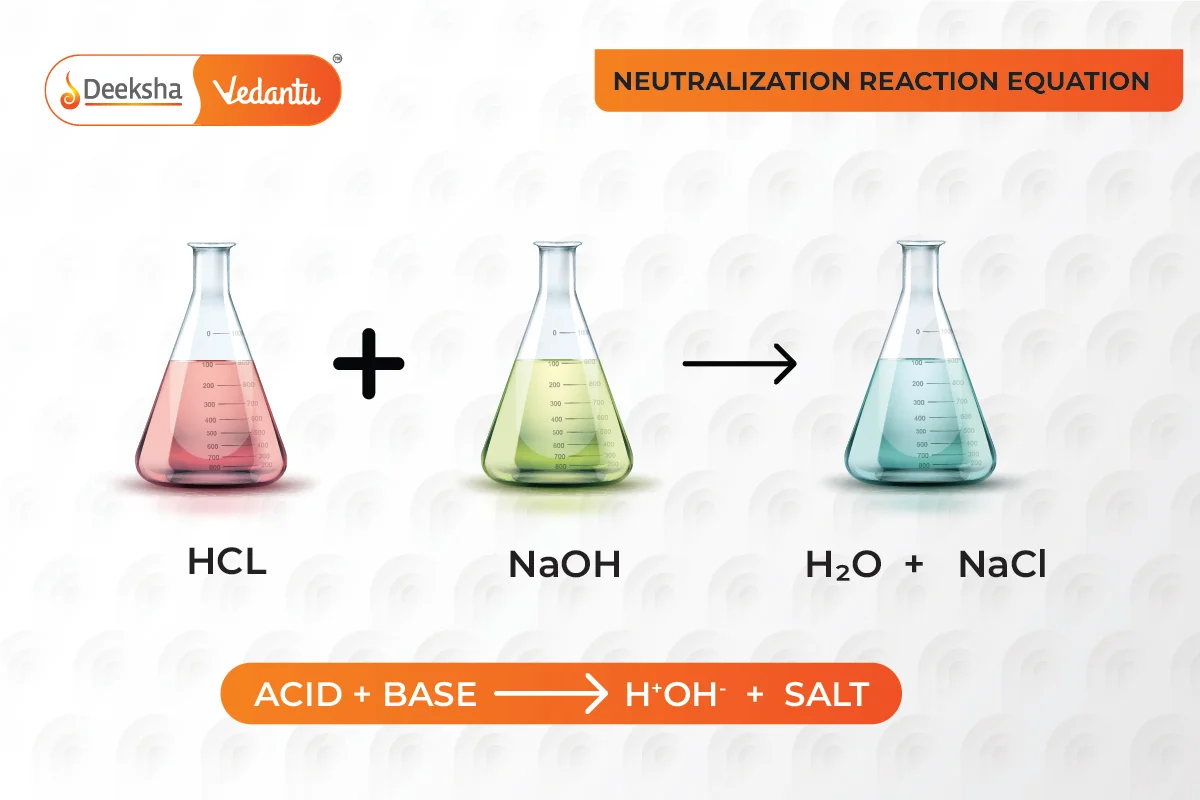
- Neutralization Reaction:
- Acid + Base → Salt + Water
- Example:
- Here, hydrochloric acid neutralizes sodium hydroxide to form sodium chloride (common salt) and water.
- Reaction with Carbonates:
- Acids react with carbonates to release carbon dioxide gas.
- Example:
pH Scale and Its Importance
The pH Scale
- Definition: The pH scale is a measure of how acidic or basic a substance is, ranging from 0 to 14.
- pH < 7: Acidic.
- pH = 7: Neutral (e.g., pure water).
- pH > 7: Basic.
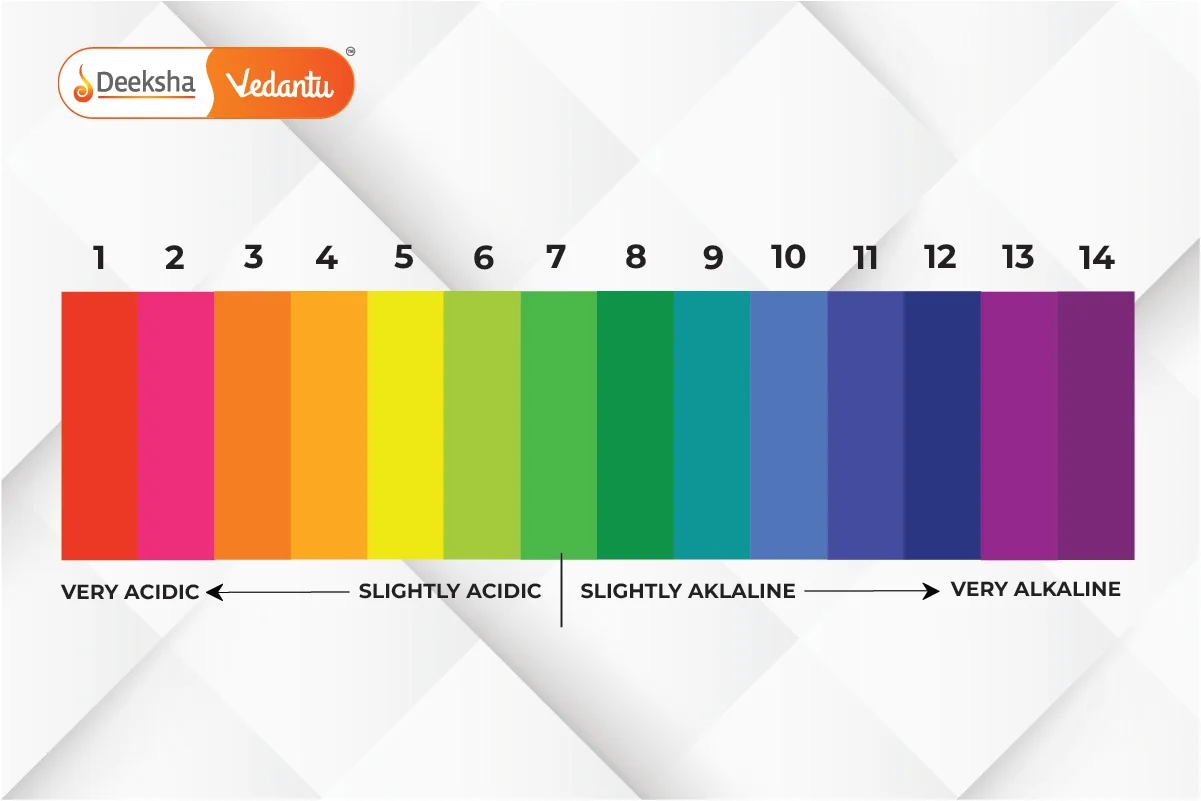
Examples of pH values:
- Lemon juice: pH ~ 2 (acidic).
- Pure water: pH = 7 (neutral).
- Baking soda: pH ~ 9 (basic).
Real-life Importance of pH:
- In Agriculture:
- The pH of soil affects plant growth. Most plants prefer neutral to slightly acidic soil.
- In the Human Body:
- The stomach produces hydrochloric acid (pH 1-3) to aid digestion. Antacids (like magnesium hydroxide) are used to neutralize excess stomach acid.
- Environmental Impact:
- Acid rain (pH below 5.6) can harm plant life and aquatic ecosystems by making water bodies too acidic for fish and other organisms.
Neutralization Reactions
Definition:
A neutralization reaction occurs when an acid reacts with a base to form salt and water. This process neutralizes the properties of both the acid and the base.
Example:
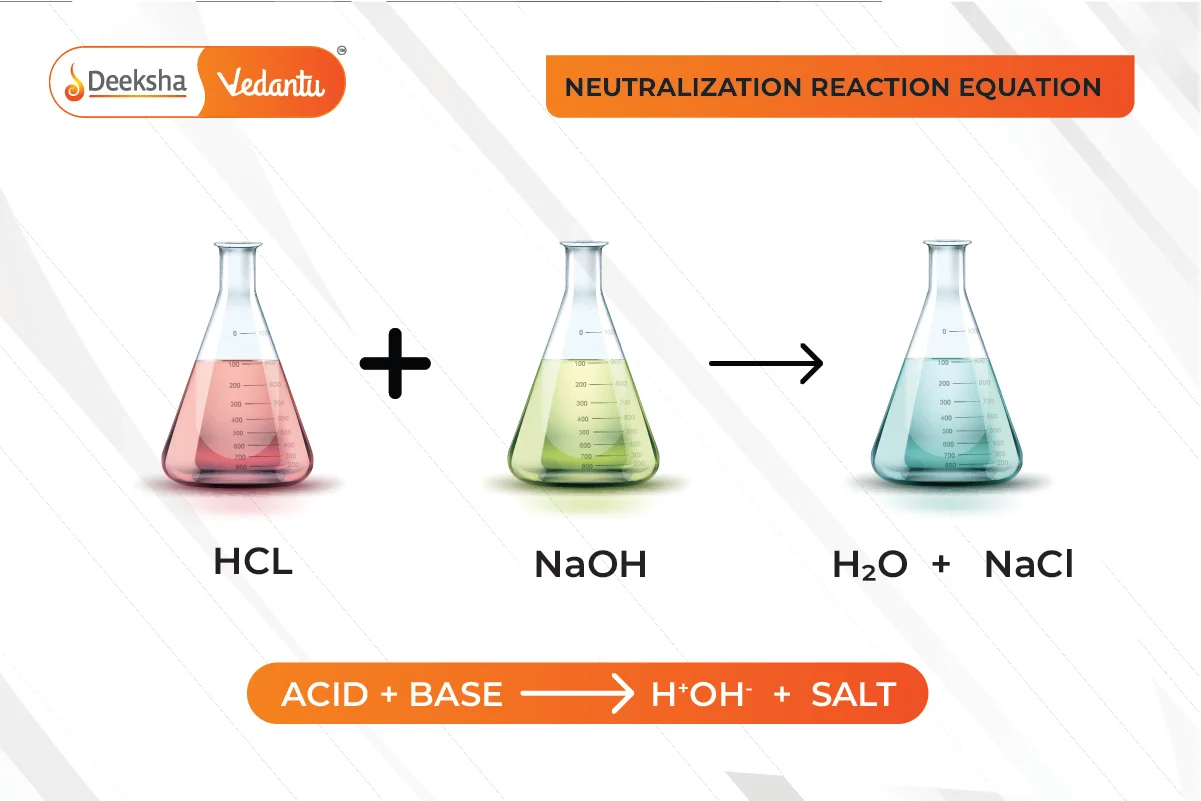
Real-life Applications:
- Medicine: Antacids neutralize excess stomach acid, providing relief from indigestion.
- Agriculture: Lime (calcium hydroxide) is used to neutralize acidic soils to make them suitable for crop growth.
Common Indicators and Their Uses
Indicators:
Indicators are substances that change color depending on the pH of the solution they are added to. They help identify whether a solution is acidic or basic.
Natural Indicators:
- Litmus:
- Red in acidic solutions, blue in basic solutions.
- Turmeric:
- Turns red in basic solutions.
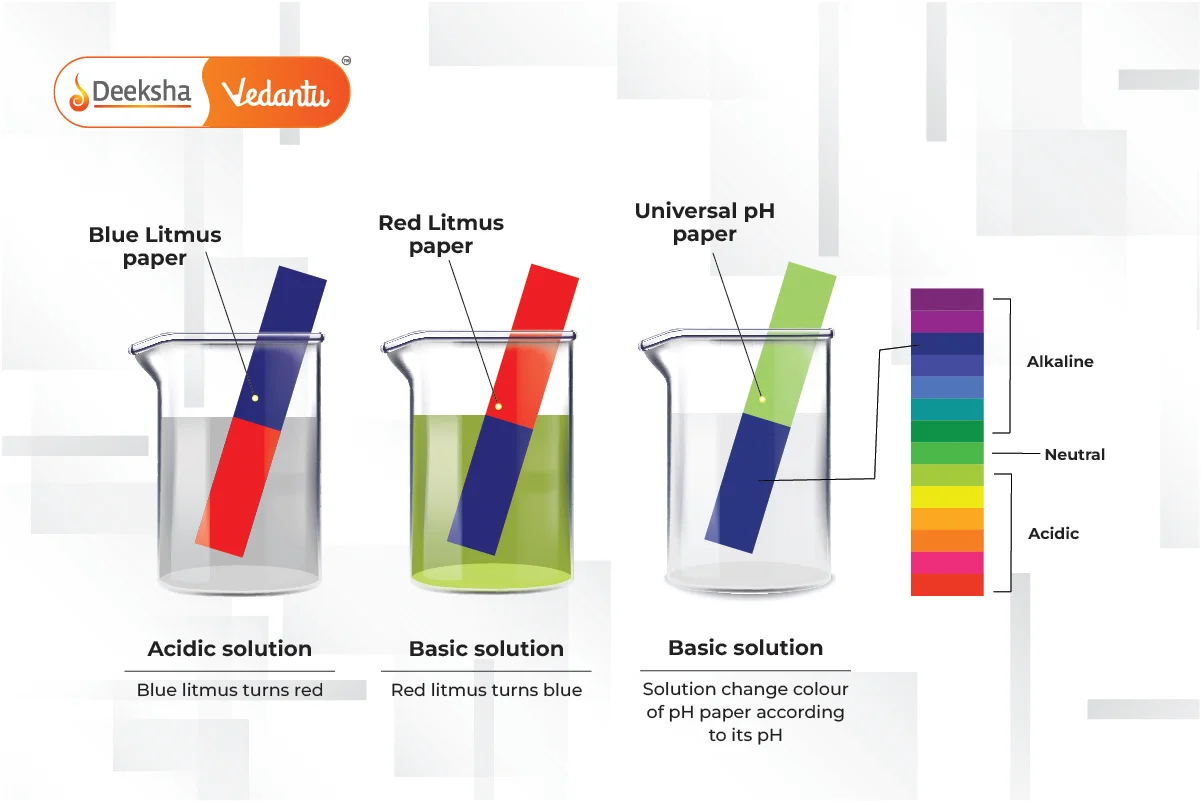
Synthetic Indicators:
- Phenolphthalein:
- Colorless in acidic solutions, pink in basic solutions.
- Methyl Orange:
- Red in acidic solutions, yellow in basic solutions.
Types of Salts
Normal Salts:
- Formed when all the hydrogen ions in the acid are replaced by metal ions.
- Example: Sodium chloride
from hydrochloric acid and sodium hydroxide.
- Example: Sodium chloride
Acidic Salts:
- Formed by the incomplete neutralization of polybasic acids.
- Example: Sodium hydrogen sulphate
.
- Example: Sodium hydrogen sulphate
Basic Salts:
- Formed by the incomplete neutralization of polyacidic bases.
- Example: Zinc hydroxide chloride
.
- Example: Zinc hydroxide chloride
Key Questions
- Explain the reaction of zinc with hydrochloric acid. Write the balanced equation.
- Answer: Zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas.
- Equation:
- Equation:
- Answer: Zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas.
- Why is the pH of water important for aquatic life?
- Answer: The pH of water affects the availability of nutrients and gases for aquatic organisms. A pH that is too low or too high can be harmful to fish and other aquatic species.
- What happens when an acid reacts with a metal carbonate?
- Answer: The reaction produces salt, carbon dioxide, and water.
- Equation:
- Equation:
- Answer: The reaction produces salt, carbon dioxide, and water.
FAQs
Antacids neutralize excess stomach acid by reacting with it to form salt and water.
pH is used to maintain soil quality, ensure safe drinking water, and manage health through the proper use of antacids.
Acids release hydrogen ions (), which react with litmus, causing it to turn red.
A neutralization reaction is when an acid reacts with a base to form salt and water.
Example:
Related Topics
- Protein Structure And Levels of Protein
- Electronic Configuration of First 30 Elements
- First 20 Elements of the Periodic Table
- Soil Pollution
- Suspension
- Rutherford’s Model of Atoms and its Limitations
- 118 Elements – Their Symbols and Atomic Number
- Carbon and its Compounds
- Bonding In Carbon – The Covalent Bond
- Some Important Carbon Compounds – Ethanol And Ethanoic Acid
- Natural Resources
- Corrosion
- Chemistry FAQs
- Bohr’s Model Of Atom
- Soaps And Detergents








Get Social