Discovery of Subatomic Particles
The concept of atomic structure has been a cornerstone in the advancement of science, especially in understanding how matter is constituted. Atoms consist of a nucleus, where positively charged protons and neutral neutrons are located, surrounded by negatively charged electrons that orbit the nucleus.
The Evolution of Atomic Theory
The journey into the atom began with Democritus’ ancient suggestion that matter was made of indivisible components. This idea was significantly refined by John Dalton in the 1800s, who posited that atoms were individual units that made up elements and were themselves indivisible. This laid the groundwork for future discoveries and helped to establish the basic principles of chemical reactions and interactions.
As science progressed, so did the understanding of the atomic structure. The 20th century saw major advancements with the identification of subatomic particles – electrons, protons, and neutrons – that fundamentally changed how scientists viewed atoms.
Breakthrough Models in Atomic Structure
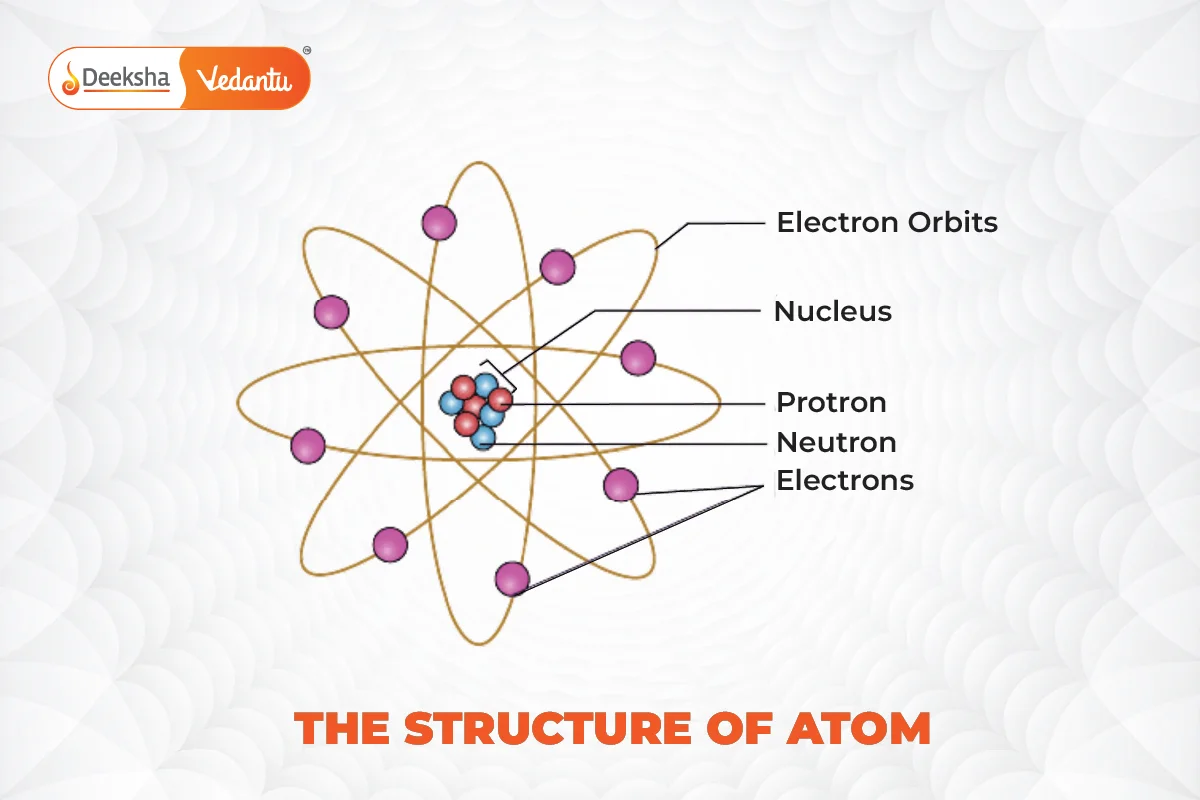
Dalton’s Atomic Model
John Dalton’s atomic model introduced the concept that each element had its own kind of atom, and these atoms could combine in certain fixed ratios to form compounds. Despite its simplicity, this model explained several laws of chemical combination and conservation of mass.
Thomson’s Plum Pudding Model
J.J. Thomson’s discovery of the electron led to his plum pudding model, where he envisioned the atom as a positively charged sphere with electrons embedded within it. His model was supported by his cathode ray experiments, which also determined the charge-to-mass ratio of the electron.
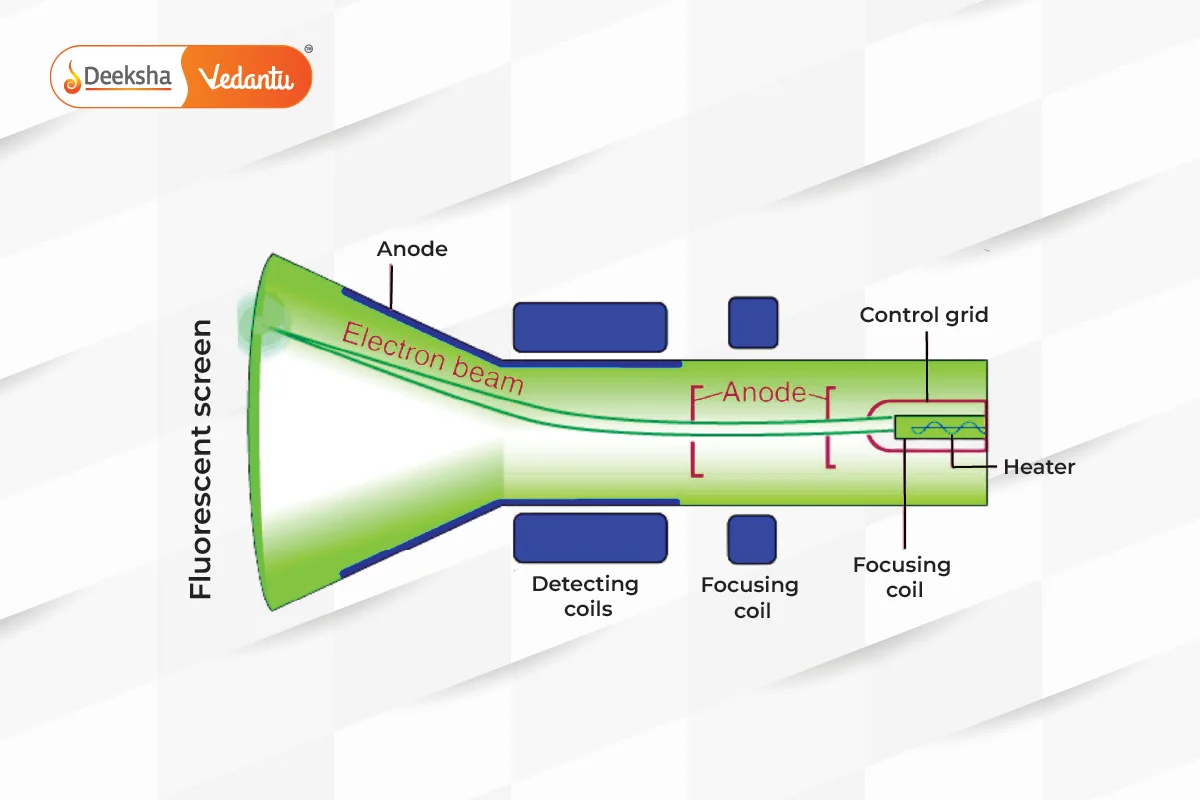
Rutherford’s Nuclear Model
Ernest Rutherford, through his gold foil experiment, discovered the nucleus of the atom. His model depicted the atom as mostly empty space, with a dense central nucleus surrounded by electrons. This model introduced the concept of the nuclear structure of the atom but struggled to explain the stability of the orbiting electrons.
Bohr’s Quantum Model
Niels Bohr further refined atomic theory by introducing quantum mechanics into the model of the atom. His theory proposed that electrons orbit the nucleus in fixed orbits and can jump between these orbits by absorbing or emitting energy, which explained the line spectra of elements.
Subatomic Particles: The Building Blocks of Matter
The discovery of protons and neutrons further deepened the understanding of atomic structure. Protons, found within the nucleus, define the atomic number and, hence, the identity of an element. Neutrons, also located in the nucleus, contribute to the mass of the atom but do not affect its electrical charge.
Electrons, while contributing minimally to the mass of an atom, play a critical role in chemical bonds and reactions. The behavior and arrangement of electrons around the nucleus define an element’s reactivity and properties.
Modern Understanding of Atomic Structure
Today, the atomic model includes a dense nucleus containing both protons and neutrons surrounded by a cloud of electrons in probabilistic locations defined by quantum mechanics. This model not only explains the behavior of atoms in various chemical reactions but also the properties of elements across the periodic table.
Isotopes and Their Applications
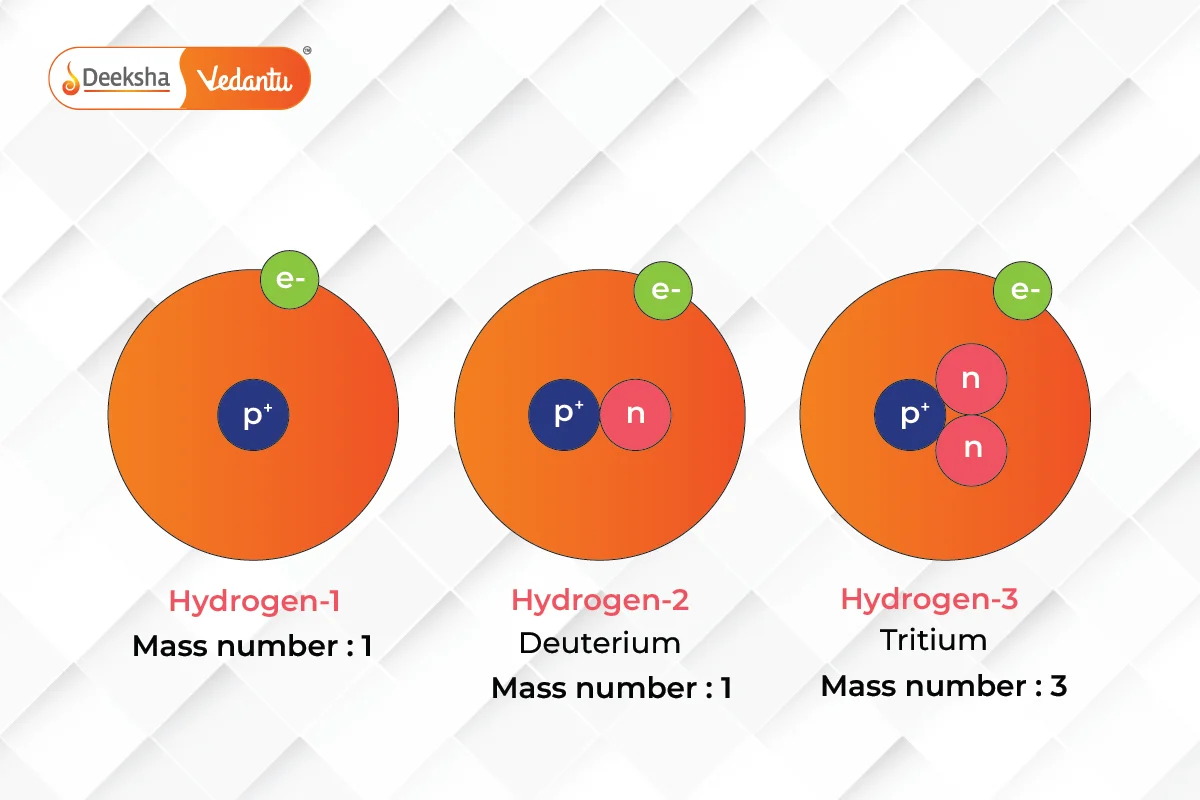
Isotopes, or variants of elements that differ in neutron number, have proven crucial in various fields such as medicine, archaeology, and environmental science. Understanding isotopes allows for the tracing of chemical and biological processes in ways that were previously impossible.
FAQs
Isotopes are crucial for various scientific applications, including medical imaging, cancer treatment, carbon dating in archaeology, and tracing environmental changes.
Bohr’s model introduced quantum mechanics into the atomic structure, proposing that electrons orbit the nucleus in fixed paths or shells and can jump between these shells by emitting or absorbing energy.
Ernest Rutherford discovered the nucleus of the atom and proposed that atoms consist mostly of empty space, with a dense central nucleus.
J.J. Thomson discovered the electron in 1897 during his experiments with cathode rays.
Atomic structure refers to the arrangement of protons, neutrons, and electrons within an atom. Protons and neutrons form the nucleus, while electrons orbit this nucleus.
Related Topics
- JEE Advanced Marks vs Ranks 2024
- Simple Harmonic Motion (SHM)
- Young’s Double Slit Experiment
- Normality
- Heisenberg Uncertainty Principle
- Semiconductors
- Correlation Coefficient
- Chemical Bonding
- Raoult’s Law
- JEE Main Syllabus 2025
- Transformer
- Coulomb’s Law
- Rank of a Matrix and Some Special Matrices
- Basic Logic Gates
- JEE Main Marks vs Rank 2024










Get Social