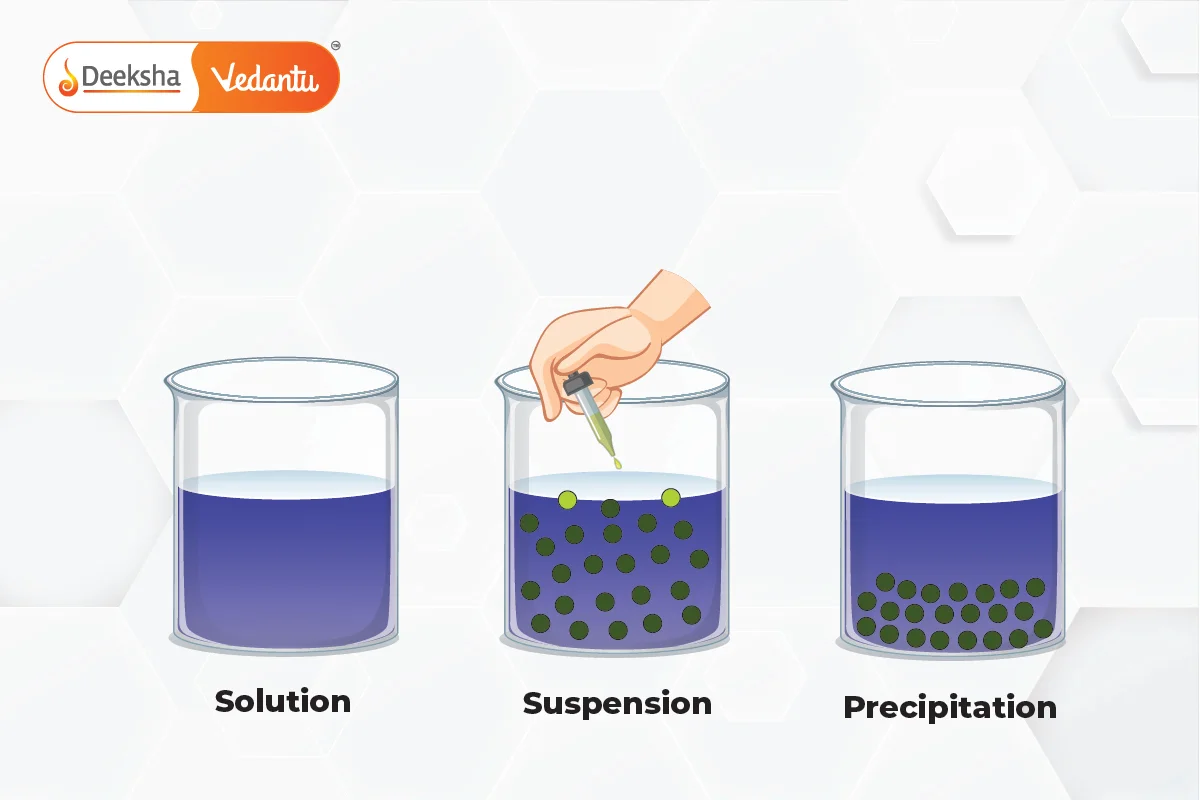
What is a Suspension?
A suspension is a heterogeneous mixture in which fine solid particles are dispersed in a liquid or gas but do not dissolve. The solid particles in a suspension are larger than in colloids or solutions and are visible to the naked eye. Over time, these particles settle at the bottom due to gravity, making suspensions unstable unless stirred or shaken continuously.
Key Characteristics of Suspensions:
- Heterogeneous nature: Suspensions consist of two or more distinct phases — a solid and a liquid or gas — where the solid is dispersed throughout the medium.
- Particle size: The particle size in a suspension typically ranges from 1 micrometer to 1000 micrometers.
- Settling: The solid particles settle at the bottom of the container if left undisturbed.
- Tyndall Effect: Suspensions can scatter light due to the larger size of the particles, making the path of light visible when it passes through the mixture.
Examples of Suspensions
Suspensions are common in everyday life. Some well-known examples include:
- Sand in water: When sand is mixed with water, it creates a suspension. The sand particles eventually settle at the bottom if not continuously stirred.
- Muddy water: Soil particles dispersed in water form a suspension. Over time, these particles settle, leaving clear water on top.
- Flour in water: Mixing flour in water creates a suspension where the flour particles are visible and can settle out.
- Chalk in water: Chalk particles in water form a suspension, which settles over time.
Types of Suspensions
Suspensions can be classified based on the medium in which the solid is dispersed or the size of the particles.
1. Based on the Dispersing Medium:
- Liquid Suspensions: Solid particles are dispersed in a liquid. Example: Mud in water.
- Gaseous Suspensions: Solid particles are dispersed in a gas. Example: Smoke in the air.
- Aerosols: A specific type of gaseous suspension where liquid droplets or solid particles are suspended in a gas. Example: Aerosol sprays.
2. Based on Particle Size:
- Coarse Suspension: Large particles (larger than 100 micrometers) that settle quickly. Example: Grains of sand in water.
- Fine Suspension: Smaller particles (1-100 micrometers) that settle slowly.
- Example: Milk of magnesia.
Properties of Suspensions
1. Heterogeneity:
Suspensions are heterogeneous mixtures, meaning that the composition is not uniform throughout. The solid particles do not dissolve but remain suspended in the liquid or gas.
2. Visibility of Particles:
The solid particles in a suspension are visible to the naked eye or can be seen under a microscope. These particles scatter light, leading to the Tyndall effect (the visible path of light through the mixture).
3. Sedimentation:
One of the main characteristics of suspensions is the tendency of solid particles to settle out over time due to gravity. For this reason, suspensions need to be shaken or stirred to maintain their uniformity.
4. Separation by Filtration:
The solid particles in suspensions can be separated from the liquid using filtration. This property distinguishes suspensions from solutions, where the solute is completely dissolved and cannot be separated by filtration.
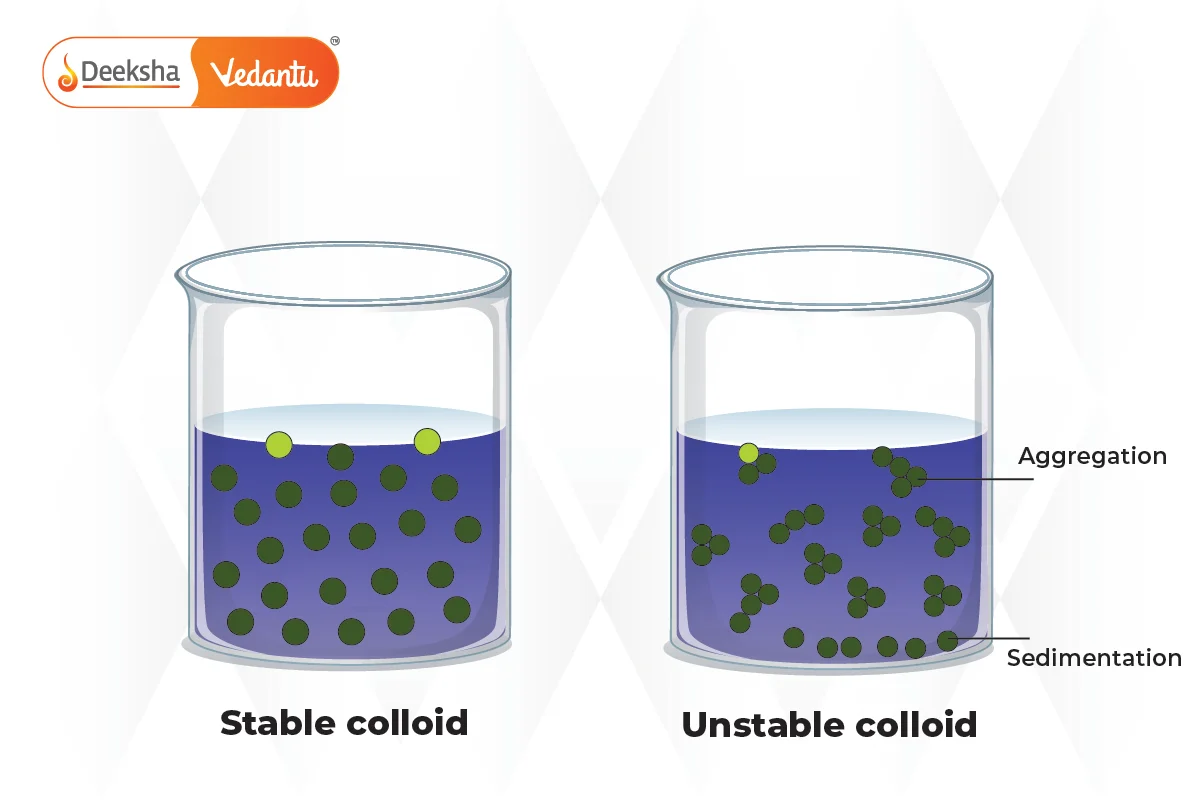
5. Instability:
Suspensions are inherently unstable mixtures. Without continuous agitation or mixing, the solid particles will settle out, forming layers in the mixture.
Difference Between Suspension, Solution, and Colloid
| Property | Suspension | Solution | Colloid |
| Definition | A heterogeneous mixture where large solid particles are dispersed in a liquid or gas but do not dissolve. Over time, the particles settle out if left undisturbed. | A homogeneous mixture where the solute is completely dissolved in the solvent at the molecular or ionic level. The particles are too small to be visible and do not settle or separate over time. | A mixture where the particle size is intermediate between a suspension and a solution. Particles do not settle out over time and scatter light, exhibiting the Tyndall effect. |
| Particle Size | Particles are larger than 1000 nm (1 micrometer). These large particles are visible to the naked eye or under a microscope. | Particles are less than 1 nm in size. These are individual molecules or ions, and are too small to be seen with the naked eye or even under a standard microscope. | Particles range from 1 nm to 1000 nm. While invisible to the naked eye, these particles are large enough to scatter light, making the mixture appear cloudy or translucent. |
| Appearance | Cloudy or opaque appearance due to the large particles suspended in the medium. Over time, the particles settle at the bottom, leading to visible separation. | Clear and transparent due to the complete dissolution of the solute in the solvent. There is no visible separation of particles. | Cloudy or translucent. The particles remain evenly dispersed throughout the medium and do not settle out, but they scatter light due to their size. |
| Homogeneity | Heterogeneous, meaning the composition of the mixture is not uniform throughout. The particles are dispersed in the medium but remain distinct. | Homogeneous, meaning the composition is uniform throughout the mixture. The solute and solvent mix completely at the molecular level, making it impossible to distinguish between them. | Heterogeneous at a microscopic level but appears homogeneous to the naked eye. Colloids have distinct phases, but the particles are too small to be visibly distinct in the mixture. |
| Stability | Unstable: The particles settle out over time due to gravity. If the suspension is not stirred, the solid particles form a sediment at the bottom of the container. | Stable: The solute does not settle out of the solution. The molecules or ions of the solute remain uniformly distributed in the solvent indefinitely. | Moderately stable: Particles do not settle out on their own, but colloids can be destabilized by adding electrolytes or using centrifugation. |
| Tyndall Effect | No: Suspensions typically do not show the Tyndall effect because the particles are too large, and instead of scattering light, they either block or transmit it irregularly. | No: Solutions do not exhibit the Tyndall effect because the solute particles are too small to scatter light. Light passes through solutions without being scattered. | Yes: Colloids exhibit the Tyndall effect, where light is scattered by the colloidal particles, making the path of light visible as it passes through the colloid. This is why colloids appear cloudy or translucent. |
| Separation by Filtration | Yes: Suspensions can be easily separated using filtration because the large particles are unable to pass through the filter paper. | No: Solutions cannot be separated by filtration because the solute particles are dissolved at the molecular level and can pass through filter paper. Distillation or evaporation may be used to separate solutes from solvents. | No: Colloidal particles are small enough to pass through filter paper. However, ultrafiltration or centrifugation can sometimes be used to separate colloidal particles from the dispersing medium. |
| Examples | Muddy water: Soil particles remain suspended in water but settle over time. Flour in water: Creates a suspension where flour particles are visible and settle at the bottom. Sand in water: Sand particles settle out. | Saltwater: Salt (NaCl) completely dissolves in water to form a clear solution. Sugar in water: Sugar dissolves to form a homogeneous solution. Air: A gaseous solution of nitrogen and oxygen. | Milk: Fat droplets dispersed in water. Fog: Water droplets dispersed in air. Whipped cream: Gas bubbles dispersed in a liquid. Blood: Cells and proteins suspended in plasma (liquid part of the blood). |
| Uses/Applications | Pharmaceutical suspensions: Certain medicines like antacids (e.g., milk of magnesia) are suspensions where the active ingredient is dispersed in liquid. Paint: Pigments suspended in liquid until applied. | Saltwater: Used in cooking, desalination, and chemistry experiments. Sugar solution: Used in beverages and syrups. Alcohol in water: Forms the basis for solutions like rubbing alcohol and hand sanitizers. | Cosmetics: Foundations and creams are colloidal mixtures. Food industry: Emulsions like mayonnaise are colloids. Medicines: Certain drugs are formulated as colloids for better absorption and efficacy. |
Applications of Suspensions
Suspensions are widely used in various industries due to their unique properties. Here are some common applications:
1. Pharmaceutical Industry:
Many medicines are formulated as suspensions, particularly for drugs that are not soluble in water. For example, antacids like milk of magnesia are suspensions. Other oral suspensions, such as antibiotics, are used when patients (especially children) cannot swallow pills.
2. Paints and Coatings:
Paints are suspensions of pigments in a liquid medium. The pigment particles remain suspended when the paint is stirred or shaken. After application, the liquid evaporates, leaving behind a solid film of paint.
3. Food and Beverage Industry:
In the food industry, suspensions are used to create products like fruit juices, which contain pulp that remains suspended in the liquid. Beverages like milkshakes or smoothies are also suspensions, where solid particles remain mixed in the liquid.
4. Water Treatment:
Suspensions play a role in water purification processes, where fine particles suspended in water are removed using filtration or settling techniques.
5. Cosmetics:
Many cosmetic products, such as foundations and creams, are suspensions of pigments and other ingredients in water or oil. These suspensions provide the desired texture and color when applied to the skin.
Preparation and Stability of Suspensions
Suspensions require careful preparation to ensure that the solid particles are evenly dispersed in the liquid or gas medium. The stability of a suspension depends on the size and density of the particles, as well as the viscosity of the medium.
Stabilizing Agents:
To improve the stability of suspensions, stabilizing agents or surfactants are often added. These agents prevent the particles from aggregating and settling quickly. In pharmaceuticals, for example, suspensions often contain thickeners or emulsifiers to ensure uniform distribution of the active ingredients.
Shaking and Stirring:
Since suspensions are inherently unstable, they need to be stirred or shaken before use to redistribute the solid particles evenly. This is especially important in medical suspensions where dosage consistency is critical.
FAQs
The Tyndall effect is the scattering of light by particles in a mixture. It occurs in suspensions due to the larger size of their particles, which scatter light.
Yes, the solid particles in a suspension can be separated by filtration, unlike solutions where the solute is dissolved.
In a suspension, the particles are large and settle over time, while in a solution, the solute is completely dissolved and does not settle out.
Stabilizing agents like surfactants or thickeners are added to prevent the solid particles from settling out too quickly.
Common examples include sand in water, muddy water, paint, and certain medicines like antacids.
A suspension is a heterogeneous mixture in which solid particles are dispersed in a liquid or gas but do not dissolve. Over time, the solid particles settle out if left undisturbed.
Related Topics
- Classification of Carbohydrates and its Structure
- Chemical Formula
- First 20 Elements of the Periodic Table
- Soaps And Detergents
- Chemical Properties Of Metals
- Reactivity Series
- Hybridization
- Versatile Nature Of Carbon
- Some Important Carbon Compounds – Ethanol And Ethanoic Acid
- Bohr’s Model Of Atom
- Understanding the Chemical Properties of Acids and Bases
- Acids, Bases, and Salts
- Electronic Configuration of First 30 Elements
- Physical Properties Of Metals And Non-Metals
- Soil Pollution











Get Social