Carbon compounds, particularly ethanol () and ethanoic acid (
), are crucial in both industrial and domestic applications. Ethanol is widely known for its use in alcoholic beverages, as a solvent, and as a biofuel, while ethanoic acid, also known as acetic acid, is the main component of vinegar and is used in various chemical and food industries.
Ethanol (C₂H₅OH)
Physical Properties of Ethanol:
- State: Liquid at room temperature.
- Boiling Point: 78°C.
- Density: 0.789 g/cm³ (at 20°C).
- Solubility: Ethanol is completely miscible with water and many organic solvents.
- Volatility: Ethanol evaporates quickly due to its relatively low boiling point.
Chemical Properties of Ethanol:
- Combustion: Ethanol undergoes combustion when exposed to oxygen, producing carbon dioxide, water, and heat energy. The reaction is highly exothermic.
- Application: Ethanol is used as a biofuel in some vehicles due to its clean-burning properties, producing fewer pollutants than gasoline.
- Reaction with Sodium: Ethanol reacts with sodium metal to form sodium ethoxide and hydrogen gas. This reaction demonstrates ethanol’s weakly acidic nature.
- Dehydration to Form Ethene: Ethanol can be dehydrated (water is removed) when heated with concentrated sulfuric acid, producing ethene (
).
- Application: Ethene is a key raw material in the plastic industry, used to make polyethylene.
Real-Life Applications of Ethanol:
- As a Solvent: Ethanol is used as a solvent in the manufacture of perfumes, paints, and drugs.
- In Beverages: Ethanol is the active ingredient in alcoholic beverages like beer, wine, and spirits.
- Disinfectant: Ethanol is a common disinfectant and is used in hand sanitizers due to its ability to kill microorganisms.
Ethanoic Acid (CH₃COOH)
Physical Properties of Ethanoic Acid:
- State: Liquid at room temperature.
- Boiling Point: 118°C.
- Density: 1.049 g/cm³.
- Solubility: Ethanoic acid is miscible with water and other organic solvents.
- Odor: Pungent, vinegar-like smell.
Chemical Properties of Ethanoic Acid:
- Reaction with Bases: Ethanoic acid reacts with bases (such as sodium hydroxide) to form salts (like sodium ethanoate) and water.
- Application: This neutralization reaction is widely used in industrial processes and in food preservation.
- Reaction with Carbonates and Bicarbonates: Ethanoic acid reacts with carbonates (e.g., sodium carbonate) and bicarbonates (e.g., sodium bicarbonate) to produce carbon dioxide, a salt, and water.
- Application: This reaction is used in the production of baked goods, where the carbon dioxide gas helps dough rise.
- Esterification: Ethanoic acid reacts with alcohols to form esters, which are sweet-smelling compounds. In the presence of concentrated sulfuric acid, ethanoic acid reacts with ethanol to form ethyl ethanoate.
- Application: Esters are used in the production of perfumes and food flavorings.
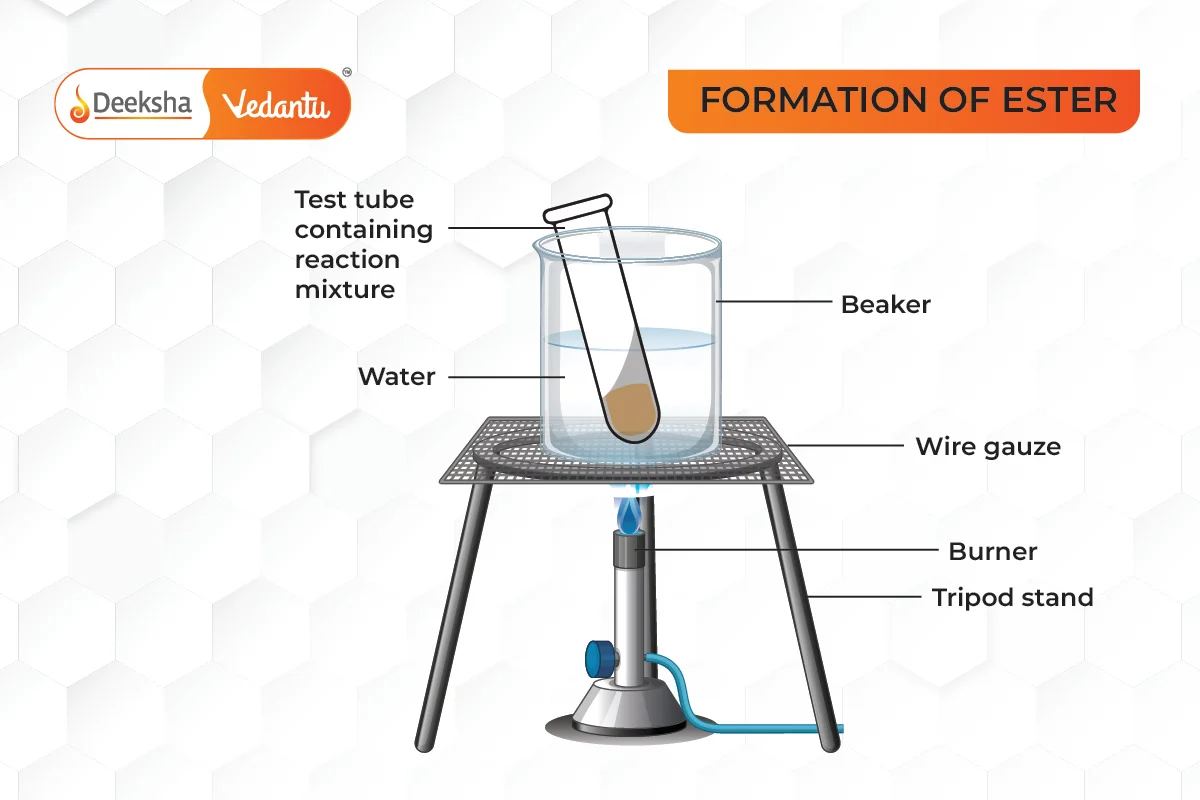
Real-Life Applications of Ethanoic Acid:
- Vinegar: Ethanoic acid is the main ingredient in vinegar, used in cooking and food preservation.
- Cleaning Agent: It is used in household cleaners to remove limescale and other mineral deposits.
- Textile Industry: Ethanoic acid is used in the production of synthetic fibers, such as cellulose acetate.
Enhanced Visualizations
- Chemical Structure of Ethanol (
)
- Ethanol is a simple alcohol with a hydroxyl group (
) attached to a two-carbon chain.
- Ethanol is a simple alcohol with a hydroxyl group (
- Chemical Structure of Ethanoic Acid (
)
- Ethanoic acid contains a carboxyl group (
) attached to a methyl group (
).
- Ethanoic acid contains a carboxyl group (
Practice Questions with Answers
Q1: Write the balanced chemical equation for the combustion of ethanol.
- Answer:
Q2: How is ethanoic acid used in household cleaning products?
- Answer: Ethanoic acid (as vinegar) is used in household cleaners to remove mineral deposits like limescale and to clean surfaces effectively.
Q3: Explain the esterification reaction between ethanoic acid and ethanol.
- Answer: Esterification occurs when ethanoic acid reacts with ethanol in the presence of concentrated sulfuric acid, forming ethyl ethanoate (an ester) and water.
FAQs
Esterification reactions produce esters, which have pleasant fragrances and are widely used in the perfume and food industries as flavoring agents.
When ethanol reacts with sodium, it forms sodium ethoxide and hydrogen gas. This reaction shows ethanol’s weakly acidic properties.
Ethanol is a renewable resource, and its combustion produces fewer pollutants compared to fossil fuels, making it an eco-friendly alternative for fuel.
Related Topics
- Versatile Nature Of Carbon
- Classification of Carbohydrates and its Structure
- Atomic Mass of Elements
- Acids, Bases, and Salts
- How Do Metals and Non-Metals React?
- Hybridization
- Bonding In Carbon – The Covalent Bond
- Physical Properties Of Metals And Non-Metals
- First 20 Elements of the Periodic Table
- How Strong Are Acid Or Base Solutions?
- Carbon and its Compounds
- Chemical Formula
- Metals and Non-Metals
- Understanding the Chemical Properties of Acids and Bases
- Modern Periodic Table







Get Social