What is Chemical Bonding?
Chemical bonding refers to the attractive force that binds atoms, molecules, or ions together to form chemical compounds. These bonds are crucial for the stability and existence of compounds. The stability of a chemical compound depends significantly on the strength of these bonds.
The fundamental concept behind chemical bonding is the stabilization of atoms through energy loss. Atoms tend to form bonds to achieve a more stable electron configuration, usually by losing, gaining, or sharing electrons. When attractive forces between atoms decrease energy, they form stable bonds; conversely, repulsive forces increase energy and destabilize the compound.
Important Theories on Chemical Bonding
Lewis Theory of Chemical Bonding
- Structure of Atom: Lewis proposed that an atom consists of a positively charged core (nucleus plus inner electrons) and an outer shell that can hold up to eight electrons.
- Octet Rule: Atoms are most stable when they have eight electrons in their outer shell, resembling the electron configuration of noble gases.
- Formation of Bonds: Atoms achieve stability by forming chemical bonds through electron sharing (covalent bonds) or electron transfer (ionic bonds).
Kossel’s Theory of Chemical Bonding
- Electrostatic Attraction: Kossel emphasized the role of electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions) in forming ionic bonds.
- Noble Gas Configuration: He noted that ions tend to achieve a stable noble gas electron configuration through electron transfer.
Types of Chemical Bonds
Ionic Bonding
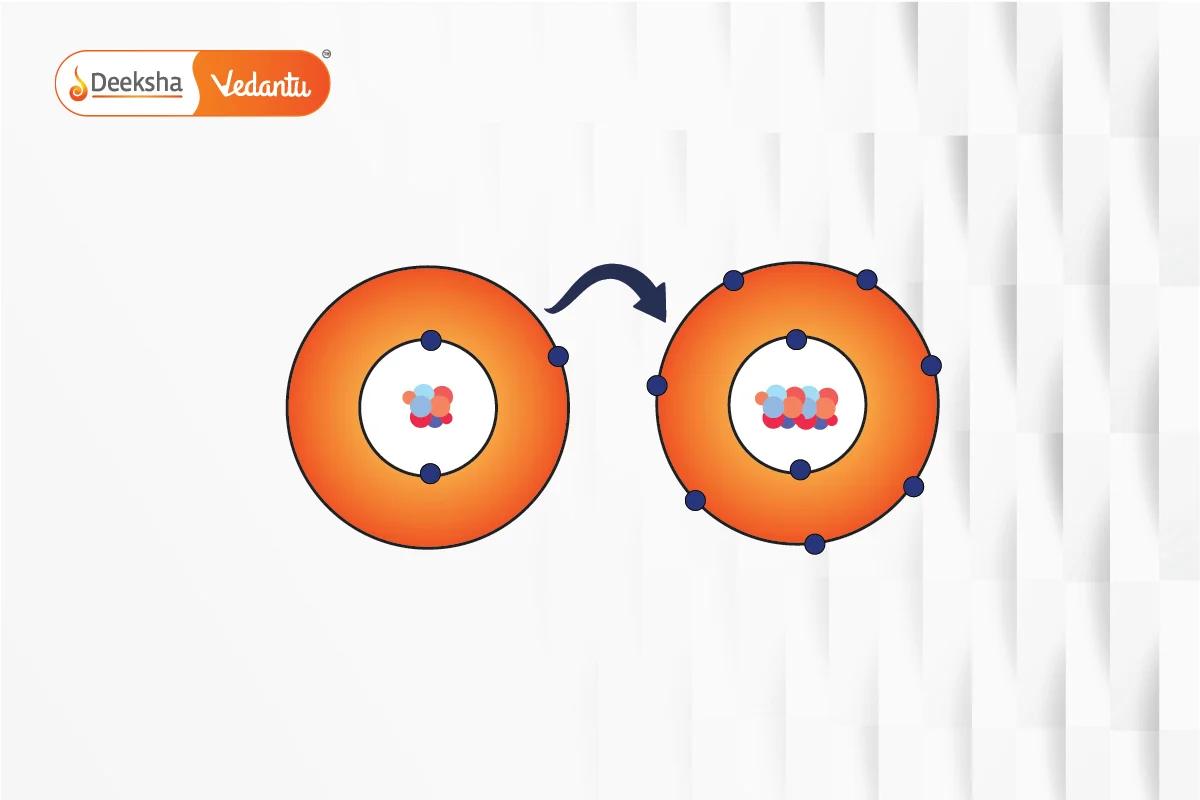
Ionic bonds form through the transfer of electrons from one atom to another, resulting in the formation of cations and anions. The electrostatic attraction between these oppositely charged ions holds them together. For example, sodium chloride is formed by the transfer of an electron from sodium to chlorine.
Covalent Bonding
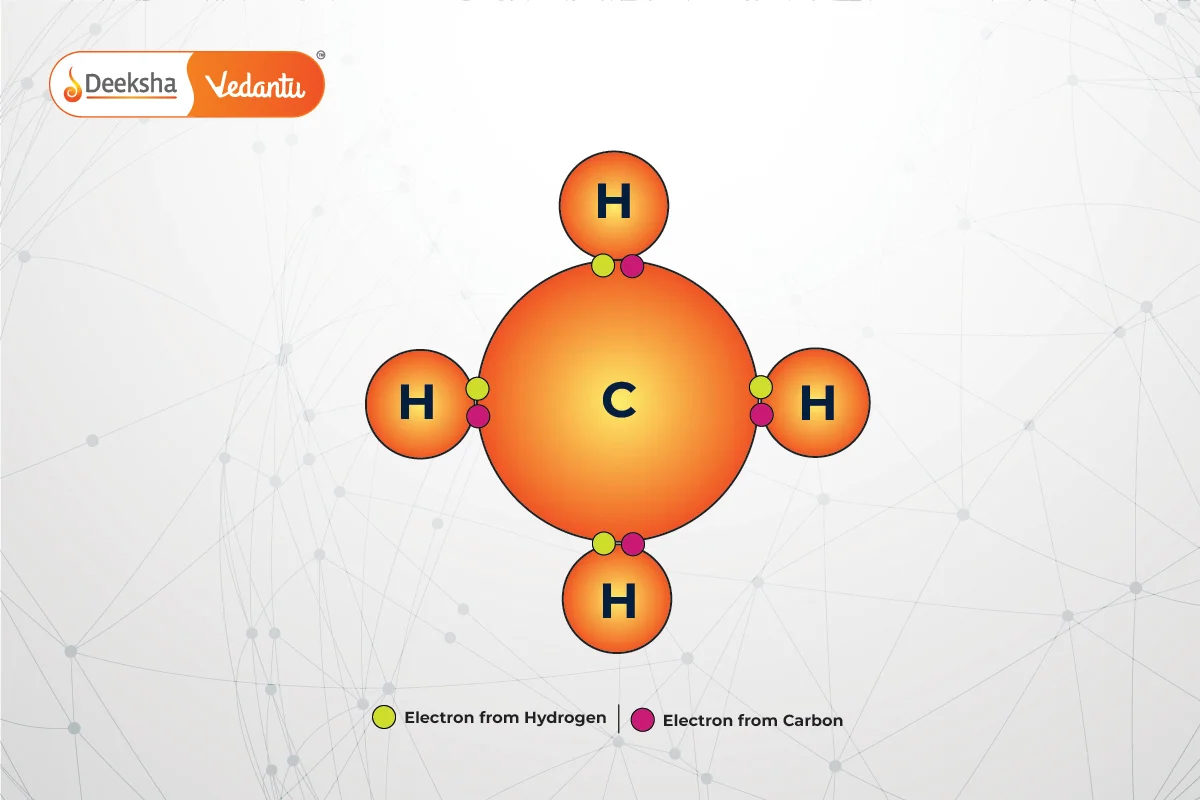
Covalent bonds involve the sharing of electron pairs between atoms. These shared electrons contribute to each atom’s octet, providing stability. Covalent bonding is common in organic compounds, such as methane .
Polar Covalent Bonding
In polar covalent bonds, electrons are shared unequally between atoms due to differences in electronegativity. This results in partial charges on the atoms, as seen in water .
Hydrogen Bonding
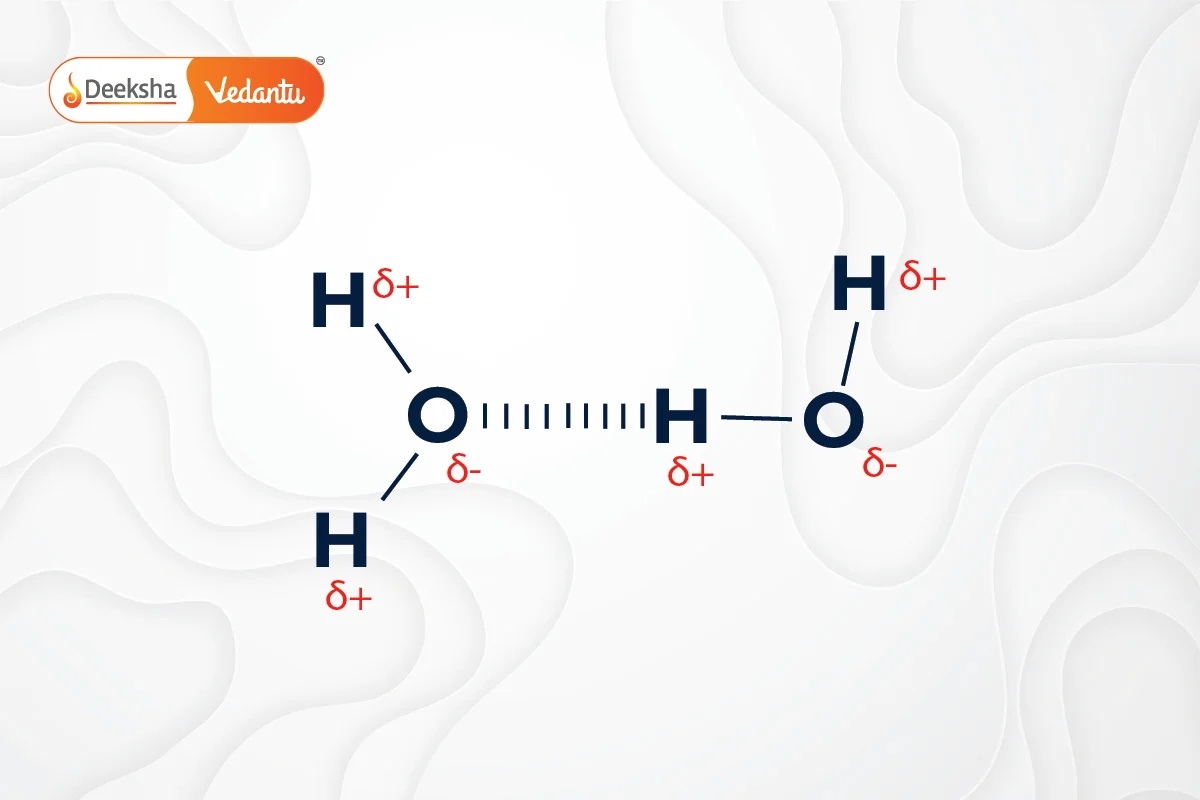
Hydrogen bonds are a type of weak chemical bond that occurs when a hydrogen atom, covalently bonded to an electronegative atom like oxygen, is attracted to another electronegative atom. This bonding is crucial in biological molecules, such as DNA.
Writing Lewis Structures
To draw Lewis structures, follow these steps:
- Determine the Total Number of Valence Electrons: Sum the valence electrons of all atoms involved.
- Construct a Skeletal Structure: Arrange the atoms, placing the least electronegative atom in the center.
- Distribute Electrons: Place electron pairs between atoms to form bonds, then distribute remaining electrons to satisfy the octet rule.
Example 1: Carbon Monoxide (CO)
- Total valence electrons:
- Skeletal structure:
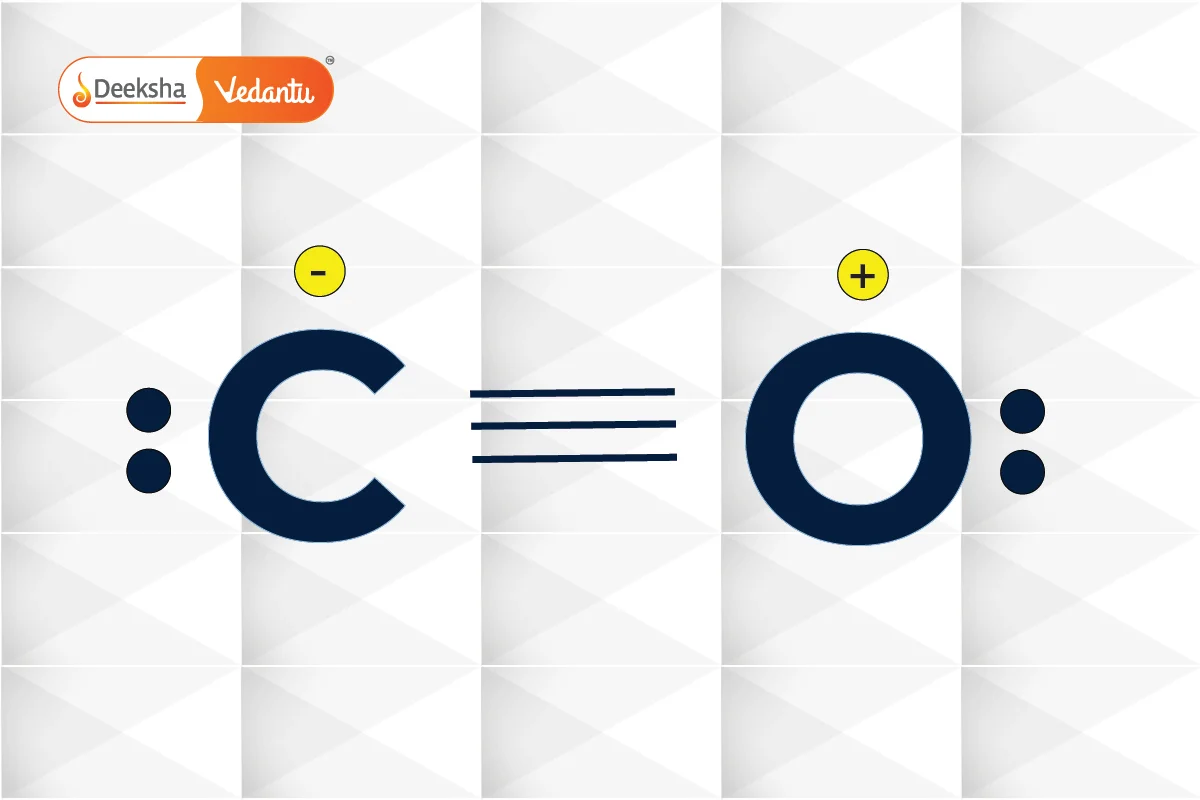
- Complete the octet: Triple bond between
and
to satisfy the octet rule.

Bond Characteristics
- Bond Length: The equilibrium distance between the nuclei of two bonded atoms. It is influenced by the size of the atoms, bond multiplicity, and the type of hybridization.
- Bond Enthalpy: The energy required to break one mole of bonds in a compound. Higher bond enthalpy indicates a stronger bond.
Resonance in Chemical Bonding
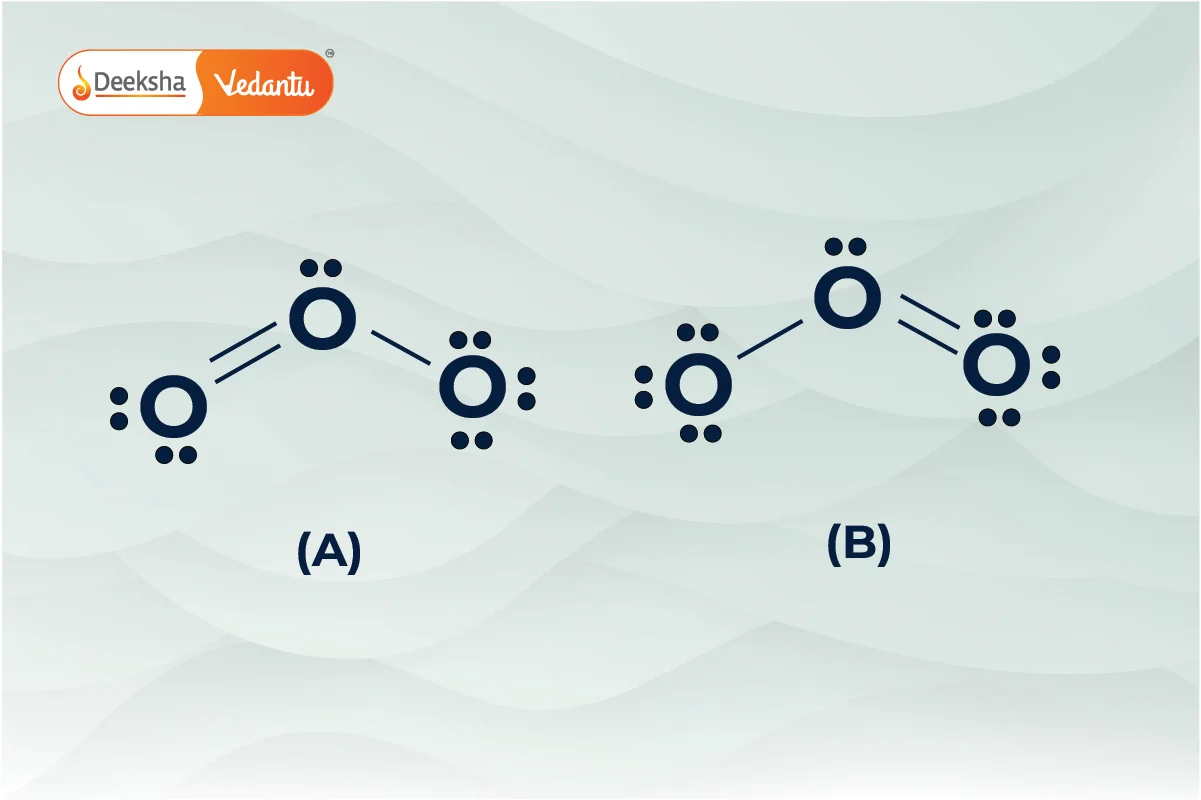
Resonance occurs when more than one valid Lewis structure can represent a molecule. The actual structure is a hybrid of these resonance forms, providing extra stability. For example, ozone exhibits resonance with two structures where the bonding pair of electrons is delocalized over all three oxygen atoms.
London Dispersion Forces
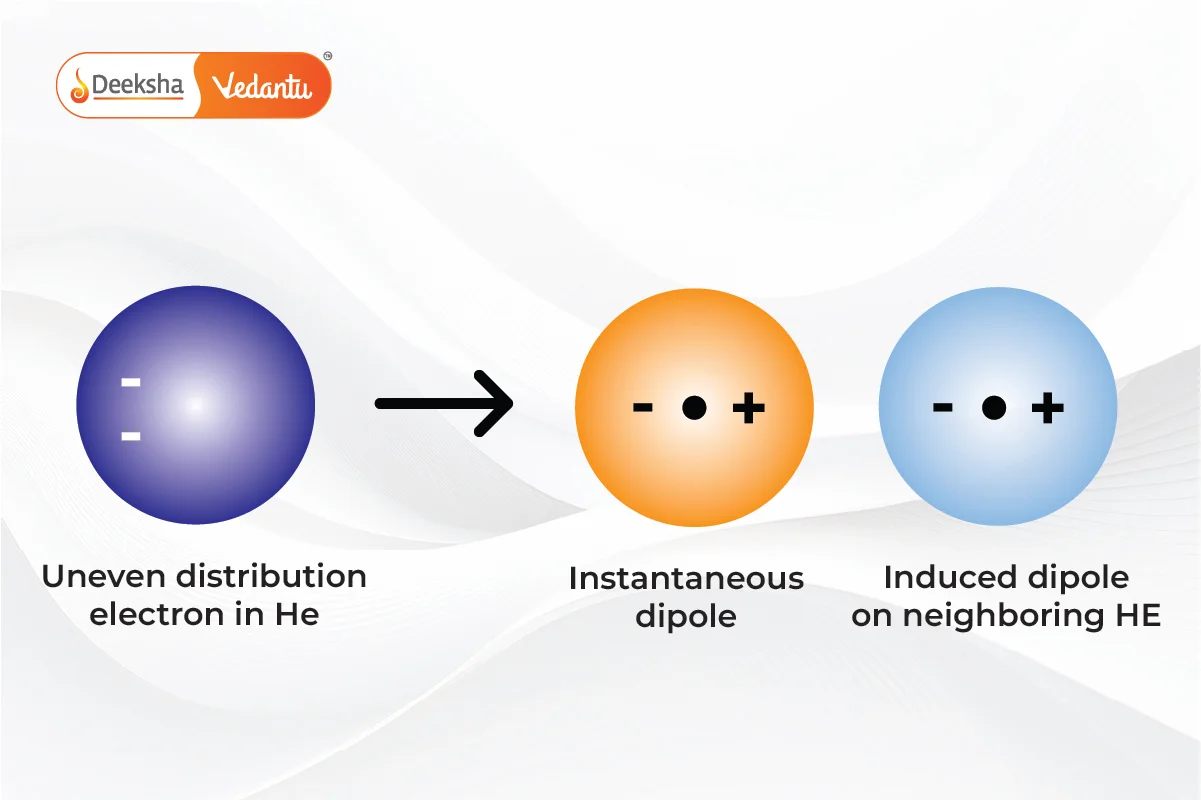
These weak intermolecular forces arise from temporary dipoles induced in atoms or molecules. They play a significant role in the physical properties of nonpolar substances.
FAQs
Resonance describes the situation where more than one valid Lewis structure can represent a molecule, indicating delocalized electrons.
Lewis structures represent the valence electrons of atoms, showing how they share or transfer electrons to form bonds.
They are weak intermolecular forces caused by temporary dipoles in atoms or molecules, significant in nonpolar substances.
Bond enthalpy is the energy required to break one mole of a specific type of bond in a compound.
The main types are ionic bonds, covalent bonds, polar covalent bonds, and hydrogen bonds.
Chemical bonding refers to the attractive force that holds atoms, molecules, or ions together in a compound.
Related Topics
- Coulomb’s Law
- Basic Logic Gates
- JEE Advanced Marks vs Ranks 2024
- Semiconductors
- Raoult’s Law
- Heisenberg Uncertainty Principle
- Normality
- Young’s Double Slit Experiment
- Atomic Structure
- Electromagnetic Spectrum
- Binomial Theorem
- JEE Main Syllabus 2025
- Rank of a Matrix and Some Special Matrices
- JEE Main Marks vs Rank 2024
- Simple Harmonic Motion (SHM)











Get Social