What is the Reactivity Series?
The reactivity series, or activity series, is a list of metals arranged in order of their reactivity, from most reactive to least reactive.
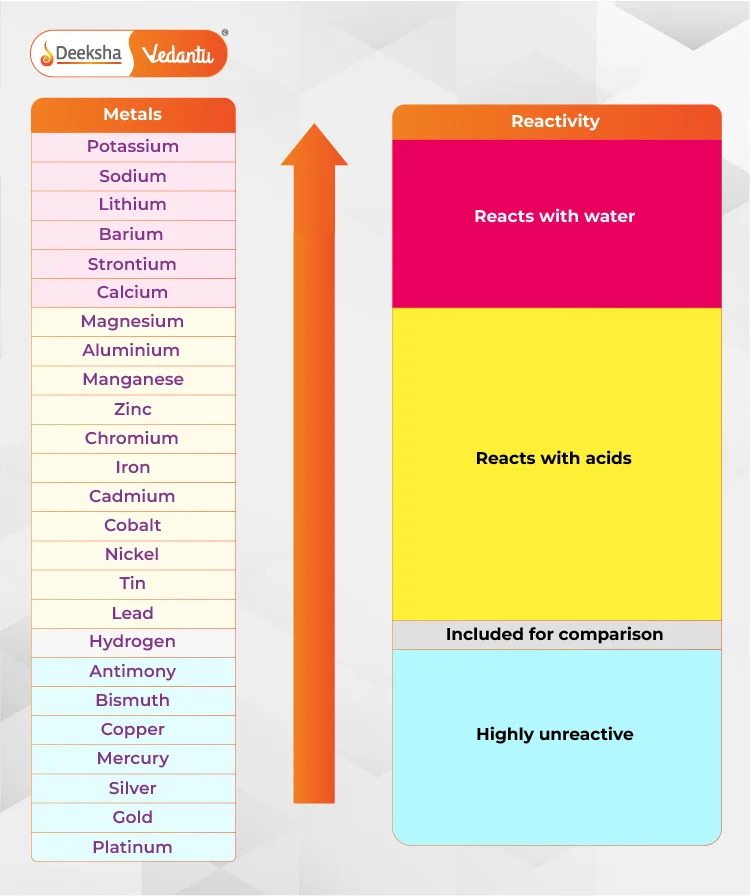
Key Features of the Reactivity Series
- Metals at the top are very reactive and easily corrode.
- Metals lower in the series are less reactive.
- Metals above hydrogen in the series can displace hydrogen from acids.
- Higher metals in the series can displace lower metals from their compounds.
- More reactive metals need more energy to be extracted from their ores.
Reactivity Series Chart
Here’s a list of metals from most reactive to least reactive:
- Caesium (Cs)
- Francium (Fr)
- Rubidium (Rb)
- Potassium (K)
- Sodium (Na)
- Lithium (Li)
- Barium (Ba)
- Radium (Ra)
- Strontium (Sr)
- Calcium (Ca)
- Magnesium (Mg)
- Beryllium (Be)
- Aluminium (Al)
- Titanium (Ti)
- Manganese (Mn)
- Zinc (Zn)
- Chromium (Cr)
- Iron (Fe)
- Cadmium (Cd)
- Cobalt (Co)
- Nickel (Ni)
- Tin (Sn)
- Lead (Pb)
- Hydrogen (H) – reference point
- Antimony (Sb)
- Bismuth (Bi)
- Copper (Cu)
- Tungsten (W)
- Mercury (Hg)
- Silver (Ag)
- Platinum (Pt)
- Gold (Au)
Hydrogen is included as a reference point to compare metal reactivity. Metals above hydrogen can displace it from acids.
Uses of the Reactivity Series
Reactions with Water:
Metals like calcium and those above it can react with cold water to form hydroxides and release hydrogen gas. For example, potassium reacts with water to produce potassium hydroxide and hydrogen gas:
Reactions with Acids:
Metals like lead and those above it can react with hydrochloric or sulfuric acid to form salts and release hydrogen gas. For instance, zinc reacts with sulfuric acid to form zinc sulfate and hydrogen gas:
Single Displacement Reactions:
Higher-ranking metals can displace lower-ranking metals from their compounds. For example, zinc can displace copper from copper sulfate:
This principle is useful in metal extraction, such as extracting titanium using magnesium.
The reactivity series helps predict and understand the behavior of metals in various chemical reactions.
FAQs
Yes, metals high in the reactivity series, such as sodium and calcium, can react with water to form hydroxides and release hydrogen gas.
Metals above hydrogen in the reactivity series can react with acids to release hydrogen gas, while metals below hydrogen do not react with acids in this way.
Hydrogen is included in the reactivity series as a reference point. Metals above hydrogen can displace hydrogen from acids, while those below cannot.
Metals like platinum and gold are at the bottom of the reactivity series. These metals are very unreactive.
Metals like caesium, francium, and potassium are at the top of the reactivity series. These metals are highly reactive.
The reactivity series helps predict how metals will react with water, acids, and in single displacement reactions. It also indicates which metals can displace others from their compounds.
The reactivity series, also known as the activity series, is a list of metals arranged in order of their reactivity from highest to lowest.
Related Topics
- Soaps And Detergents
- Bohr’s Model Of Atom
- Isomerism
- Types of Chemical Reactions
- More About Salts
- Atomic Mass of Elements
- How Do Metals and Non-Metals React?
- Hybridization
- Physical Properties Of Metals And Non-Metals
- How Strong Are Acid Or Base Solutions?
- Chemical Formula
- Bonding In Carbon – The Covalent Bond
- What Do All Acids And All Bases Have In Common?
- Chemical Properties Of Metals
- Chemistry FAQs












Get Social