Hybridization in Chemistry refers to the process where two atomic orbitals mix to create new hybridized orbitals with different energy levels and shapes. Typically, orbitals of the same energy level participate in hybridization, though both fully-filled and half-filled orbitals can join in if they have equal energy. This concept extends from the valence bond theory, helping us understand bond formation, bond energies, and bond lengths.
What is Hybridization?
Hybridization is the process where energy from individual atomic orbitals is redistributed to form orbitals of equivalent energy when two atomic orbitals combine. This results in new hybrid orbitals, which help explain atomic bonding properties and molecular geometry. For example, in a carbon atom, the valence-shell s orbital mixes with three valence-shell p orbitals to form four equivalent sp3 hybrids, arranged tetrahedrally around the carbon atom.
Key Features of Hybridization:
- Only atomic orbitals with similar energies undergo hybridization.
- The number of hybrid orbitals formed equals the number of atomic orbitals mixed.
- Both half-filled and fully-filled orbitals with slight energy differences can participate.
- Hybridization occurs during bond formation, not in isolated gaseous atoms.
- Knowing the hybridization helps predict the shape of molecules.
- Hybrid orbitals have a larger positive lobe and a smaller negative lobe.
Types of Hybridization:
Hybridization is categorized based on the types of orbitals involved:
sp Hybridization:
- Occurs when one s and one p orbital mix to form two equivalent sp hybrid orbitals.
- Results in linear molecules with a 180° bond angle.
- Example: Beryllium compounds like BeF2 and BeH2, and compounds with carbon-carbon triple bonds like C2H2.
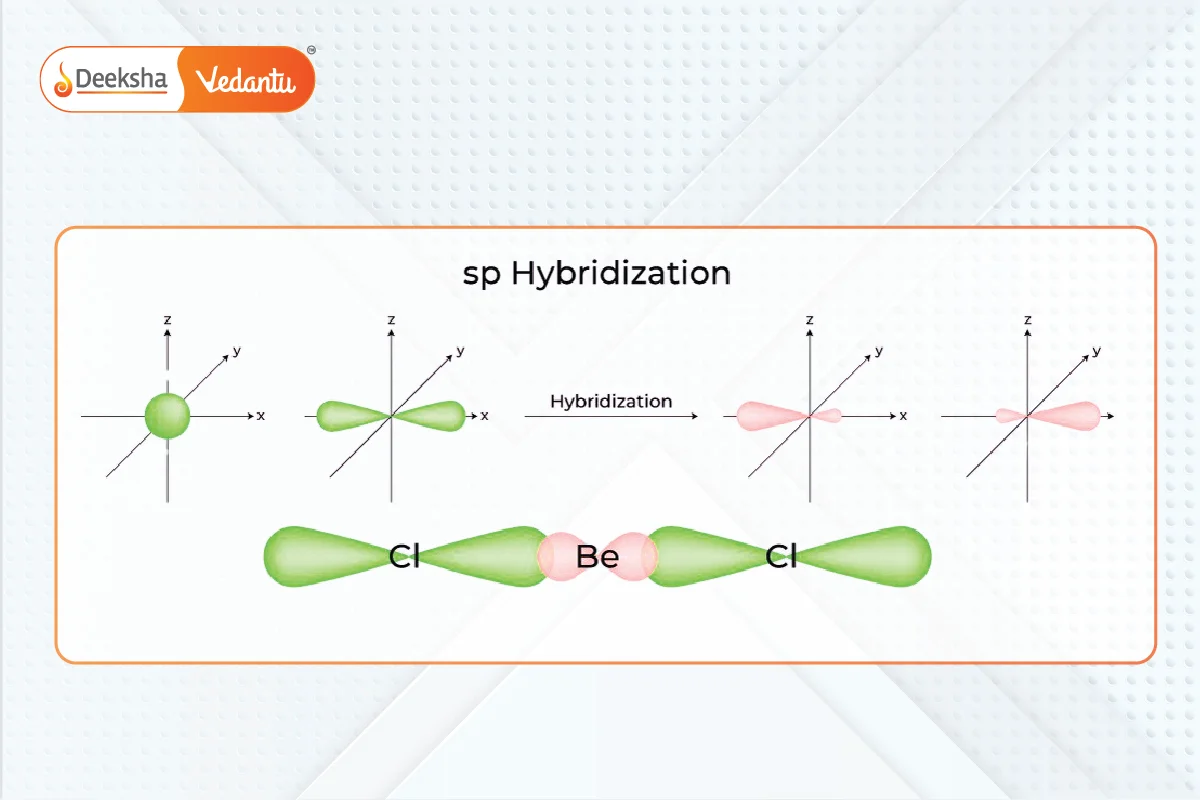
sp2 Hybridization:
- Involves one s and two p orbitals mixing to form three sp2 hybrid orbitals.
- Forms trigonal planar molecules with 120° bond angles.
- Example: Boron compounds like BF3 and BH3, and carbon compounds with double bonds like ethylene (C2H4).
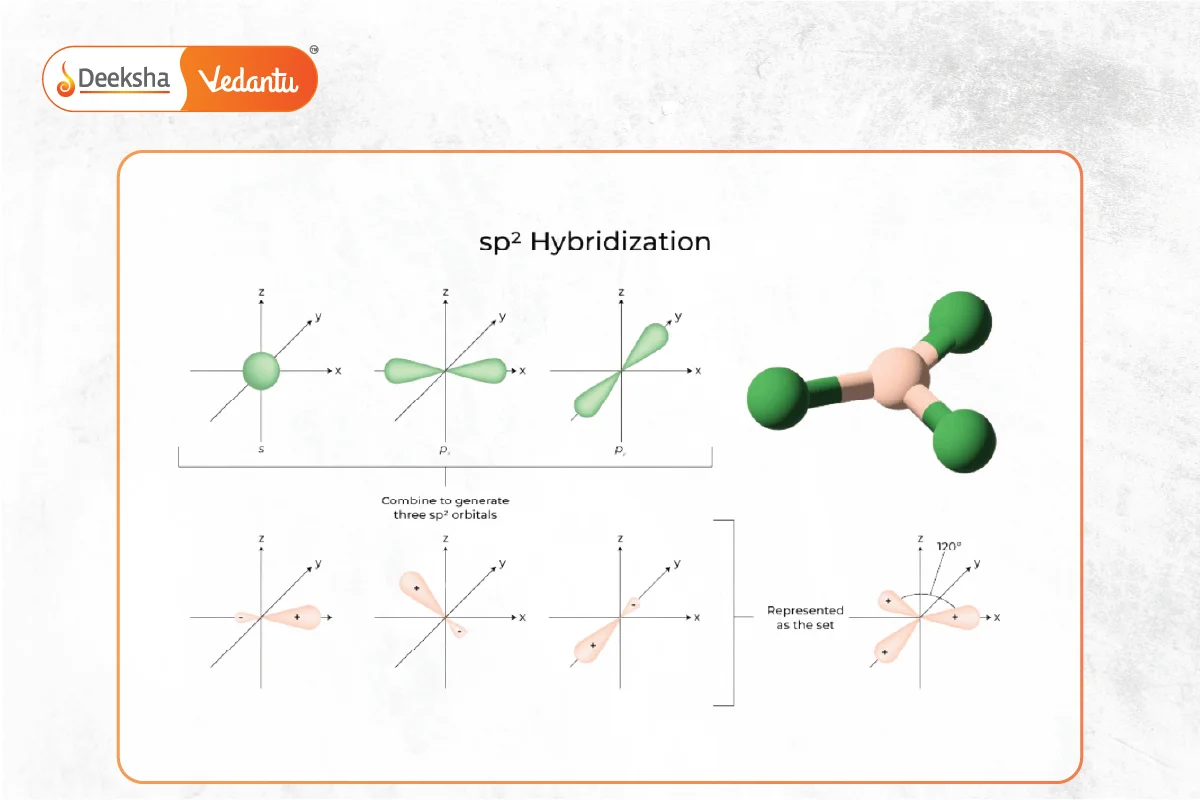
sp3 Hybridization:
- Occurs when one s and three p orbitals mix to form four sp3 hybrid orbitals.
- Forms tetrahedral molecules with 109.5° bond angles.
- Example: Methane (CH4) and ethane (C2H6).
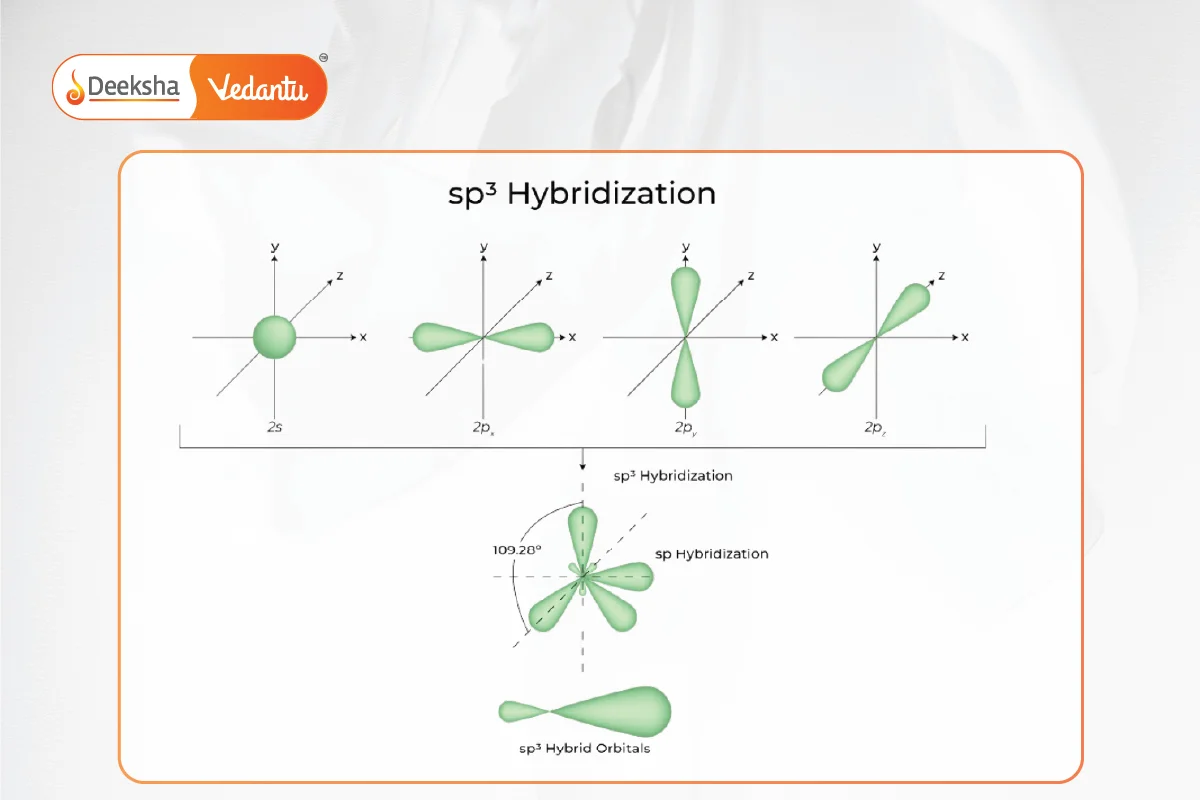
sp3d Hybridization:
- Involves mixing one s, three p, and one d orbital to form five sp3d hybrid orbitals.
- Results in trigonal bipyramidal geometry.
- Example: Phosphorus pentachloride (PCl5).
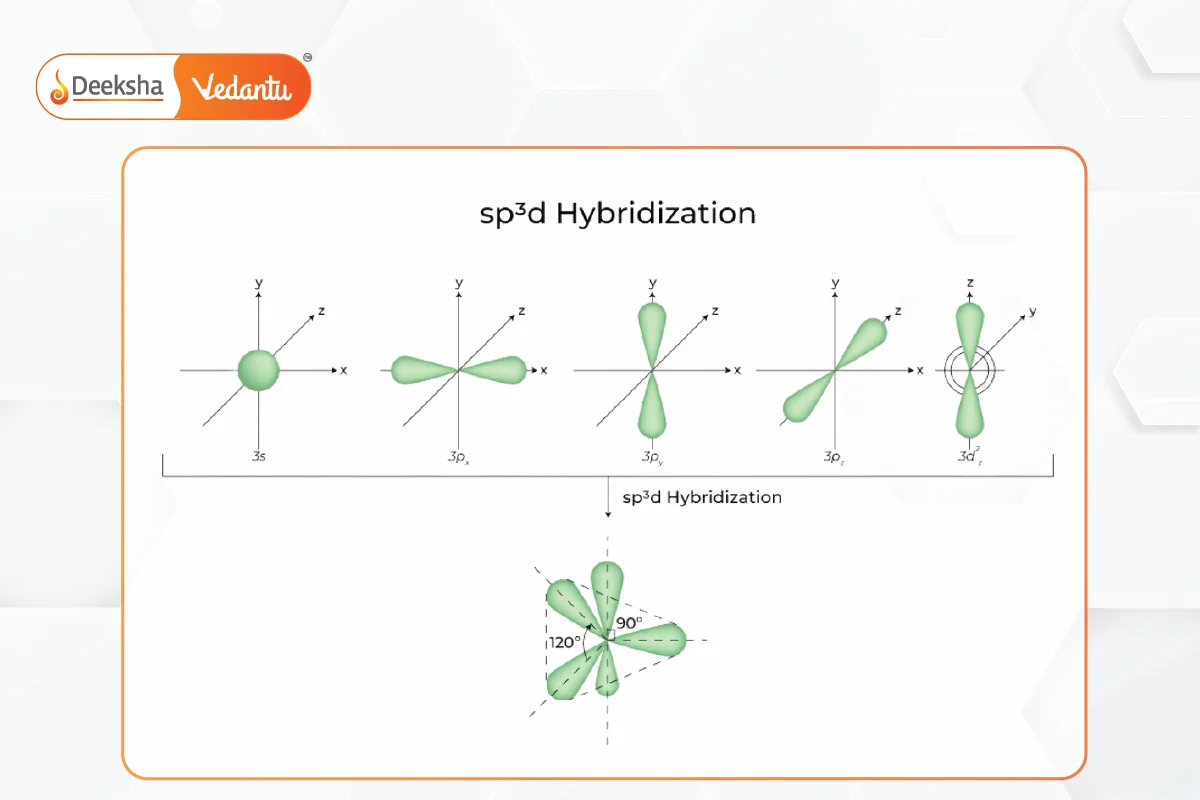
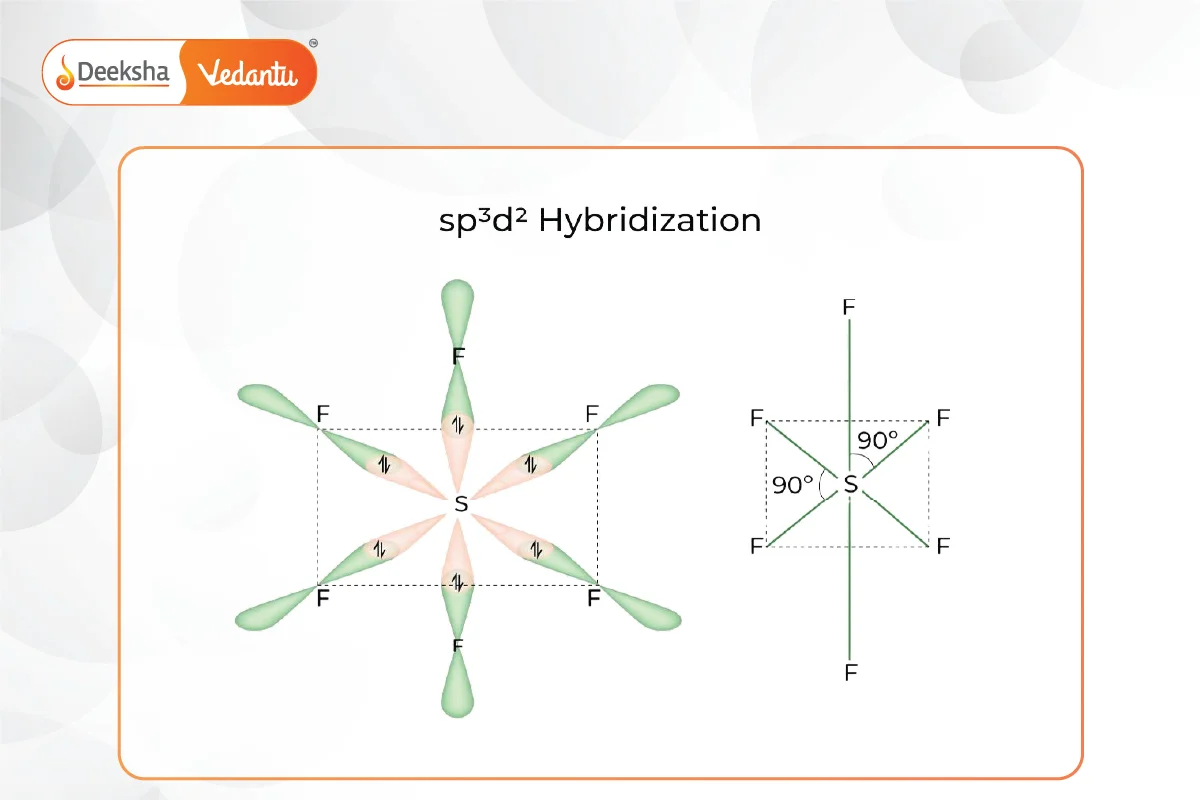
sp3d2 Hybridization:
- Involves one s, three p, and two d orbitals mixing to form six sp3d2 hybrid orbitals.
- Forms octahedral geometry with 90° bond angles.
- Example: Sulfur hexafluoride (SF6).
FAQs
Hybridization helps predict the shape and bond angles of molecules, making it easier to understand molecular geometry and bonding properties.
Yes, fully filled orbitals with slightly different energies can participate in hybridization, along with half-filled orbitals.
sp3 hybridization occurs when one s and three p orbitals mix to form four sp3 hybrid orbitals, resulting in a tetrahedral shape with 109.5° bond angles. Examples include CH4 and C2H6.
sp2 hybridization involves the mixing of one s and two p orbitals to form three sp2 hybrid orbitals, creating a trigonal planar shape with 120° bond angles. Examples include BF3 and C2H4.
sp hybridization occurs when one s and one p orbital mix to form two equivalent sp hybrid orbitals, resulting in a linear molecular shape with a 180° bond angle. Examples include BeF2 and C2H2.
There are several types of hybridization, including sp, sp2, sp3, sp3d, and sp3d2, each involving different combinations of s, p, and d orbitals.
Hybridization is the concept of mixing two atomic orbitals to create new hybrid orbitals with different energies and shapes, helping to explain atomic bonding and molecular geometry.
Related Topics
- Chemical Formula
- Differential Extraction Chromatography
- Understanding the Chemical Properties of Acids and Bases
- Bohr’s Model Of Atom
- Natural Resources
- How Strong Are Acid Or Base Solutions?
- How Do Metals and Non-Metals React?
- Carbon and its Compounds
- More About Salts
- Corrosion
- Versatile Nature Of Carbon
- Chemical Properties Of Carbon Compounds
- Soil Pollution
- Types of Chemical Reactions
- Isomerism











Get Social