What is Chromatography?
Chromatography is a technique used to separate, purify, and test compounds. The term “chromatography” comes from Greek words meaning “color” and “to write.” This technique involves applying a mixture to a stationary phase (solid or liquid) and using a solvent or gas to move the mixture over this phase, separating its components based on their solubility.
Principles of Chromatography
Chromatography separates substances in a mixture by passing it through a stationary phase with the help of a mobile phase (liquid or gas). The substances interact differently with these phases, leading to their separation. The time each substance takes to move through the stationary phase is called the retention time. The results are recorded as a chromatogram.
Types of Chromatography
There are four main types of chromatography:
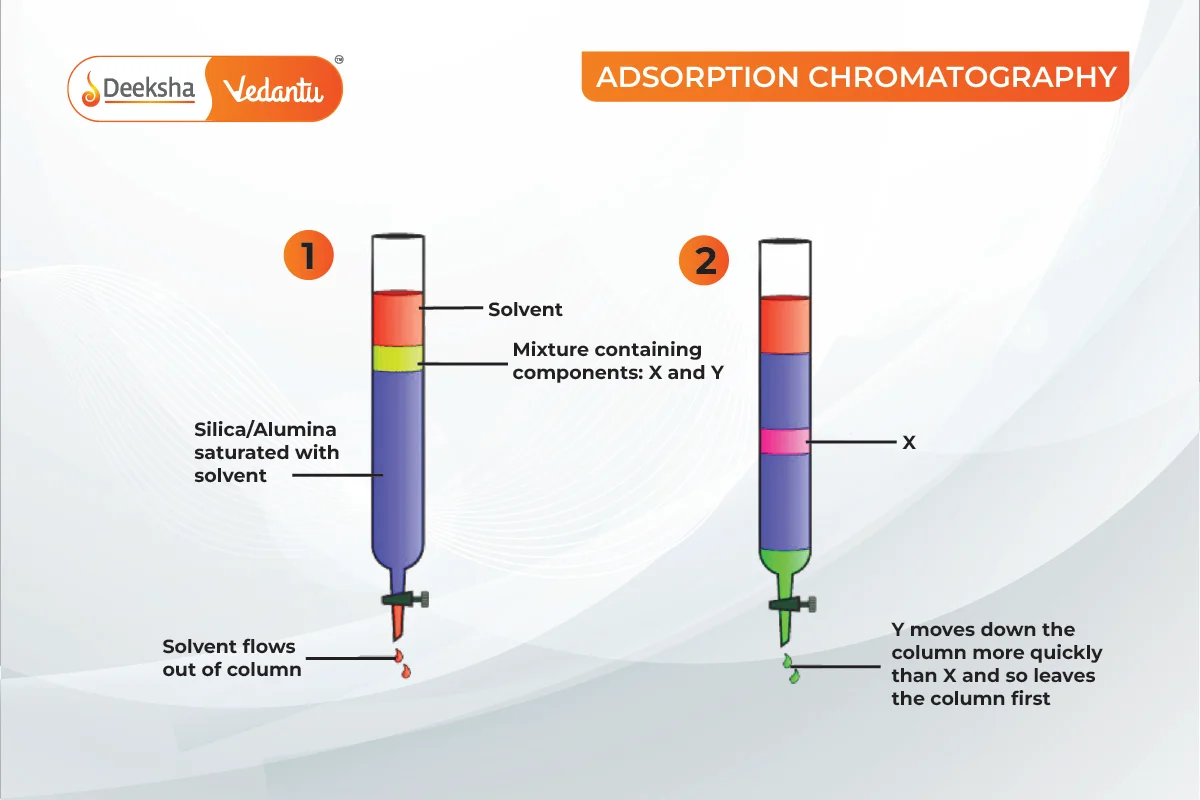
Adsorption Chromatography
Compounds are adsorbed on the adsorbent to different degrees. A mobile phase moves over a stationary phase, separating components based on their absorptivity.
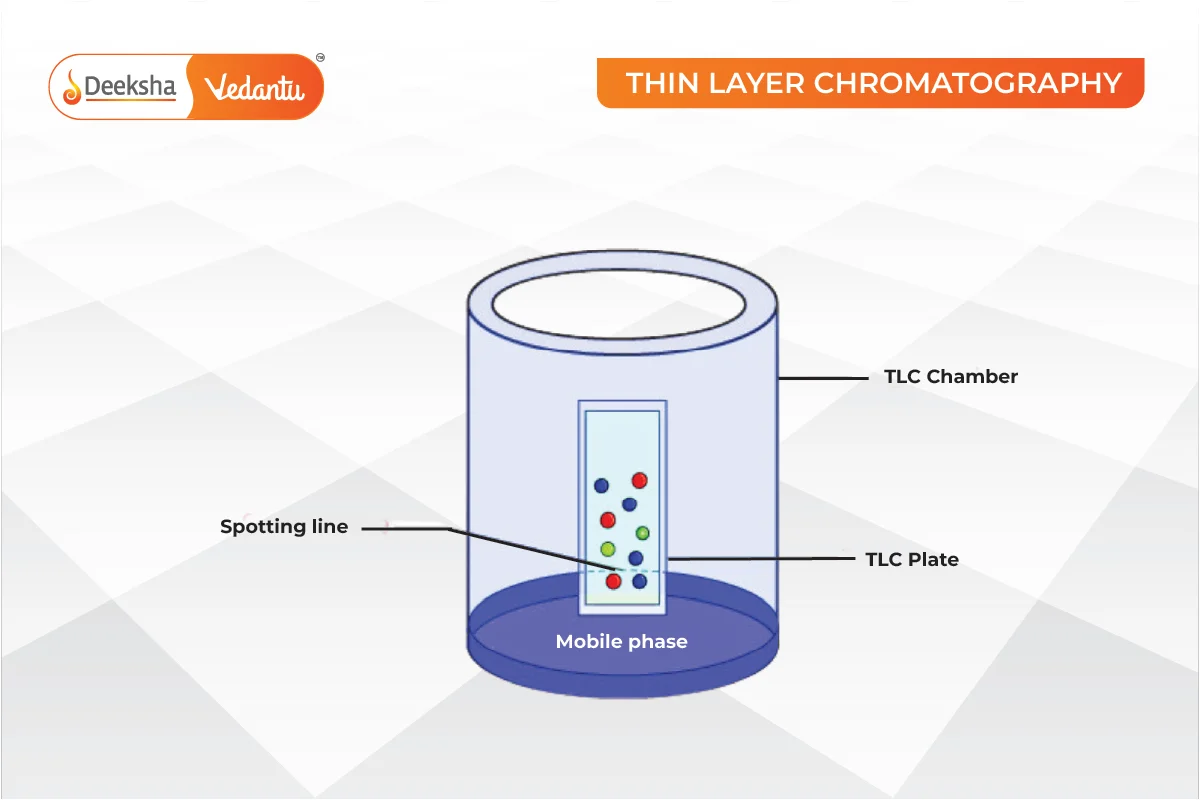
Thin Layer Chromatography (TLC)
A mixture is separated on a glass plate coated with an adsorbent like silica gel or alumina. The mixture is applied as a spot, and an eluant carries the components to different heights.
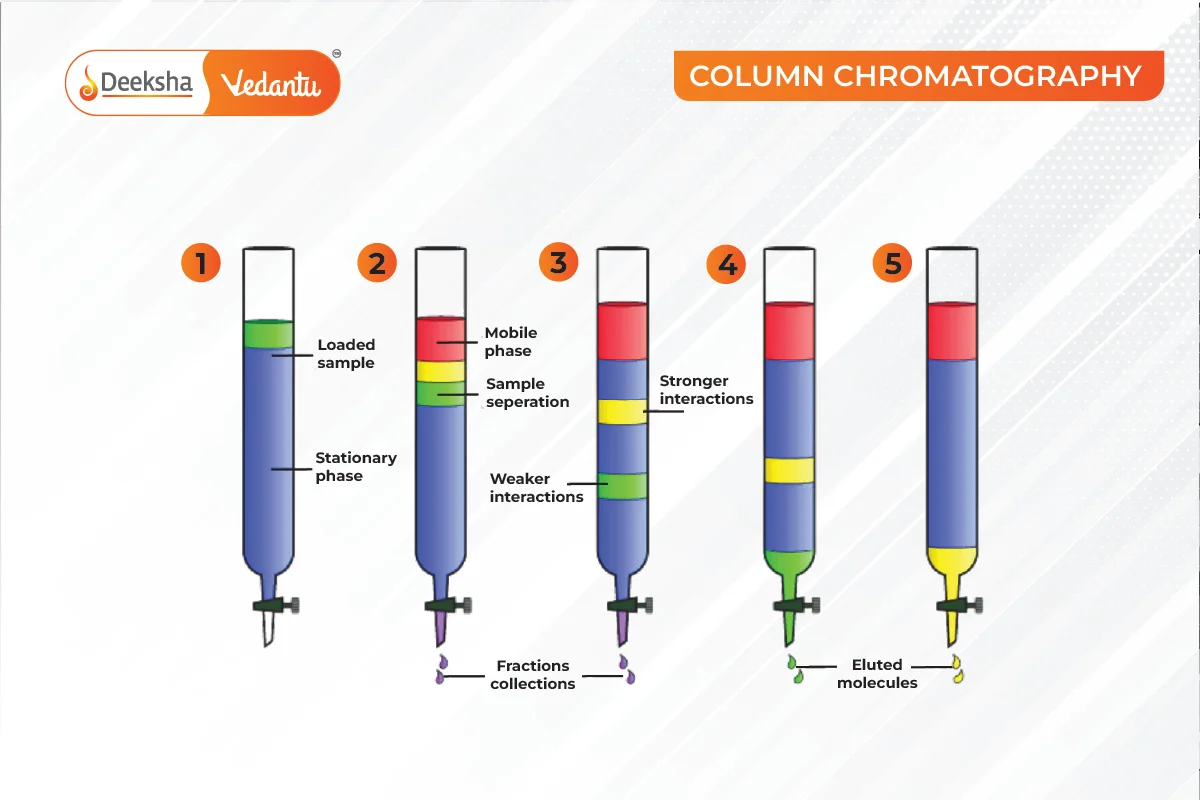
Column Chromatography
Components of a mixture are separated using a column packed with an adsorbent. The mixture is placed at the top, and an eluant flows down, separating the components based on their adsorption.
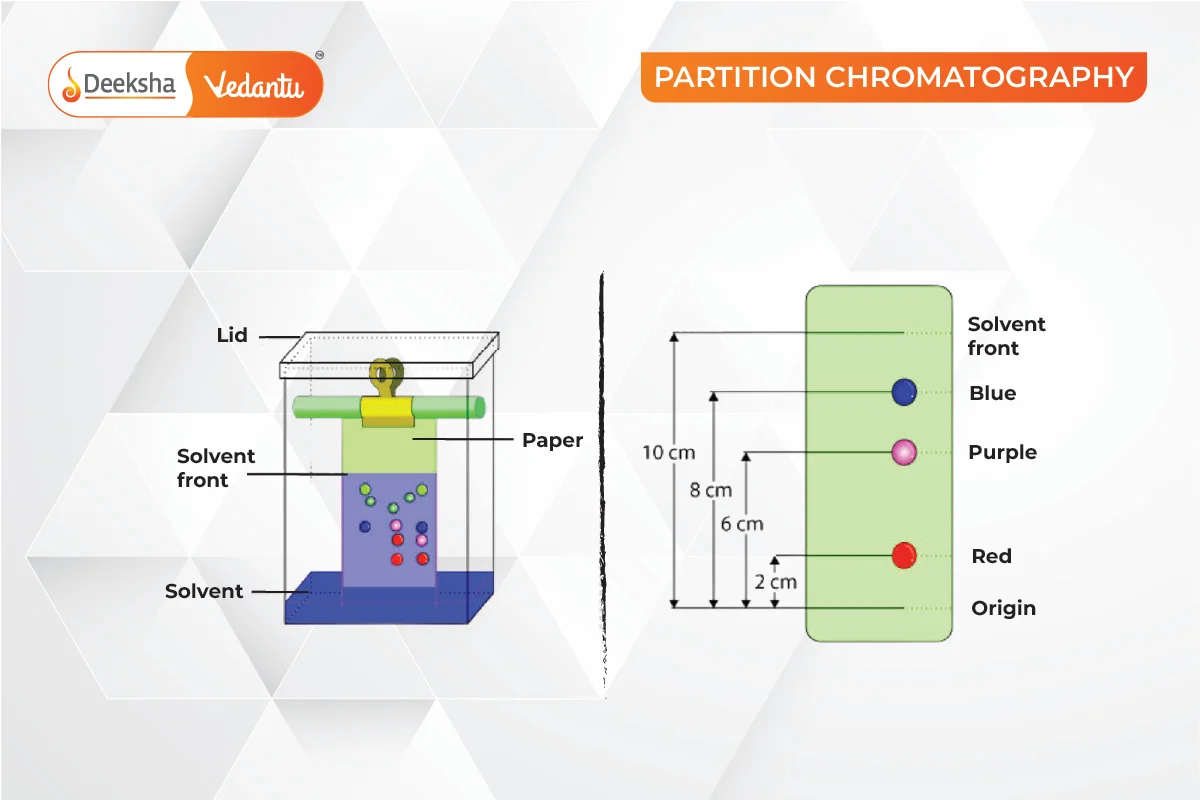
Partition Chromatography
Continuous differential partitioning occurs between a stationary phase and a mobile phase. An example is paper chromatography, where a spot of the mixture is placed on paper, and the solvent carries the components to different heights.
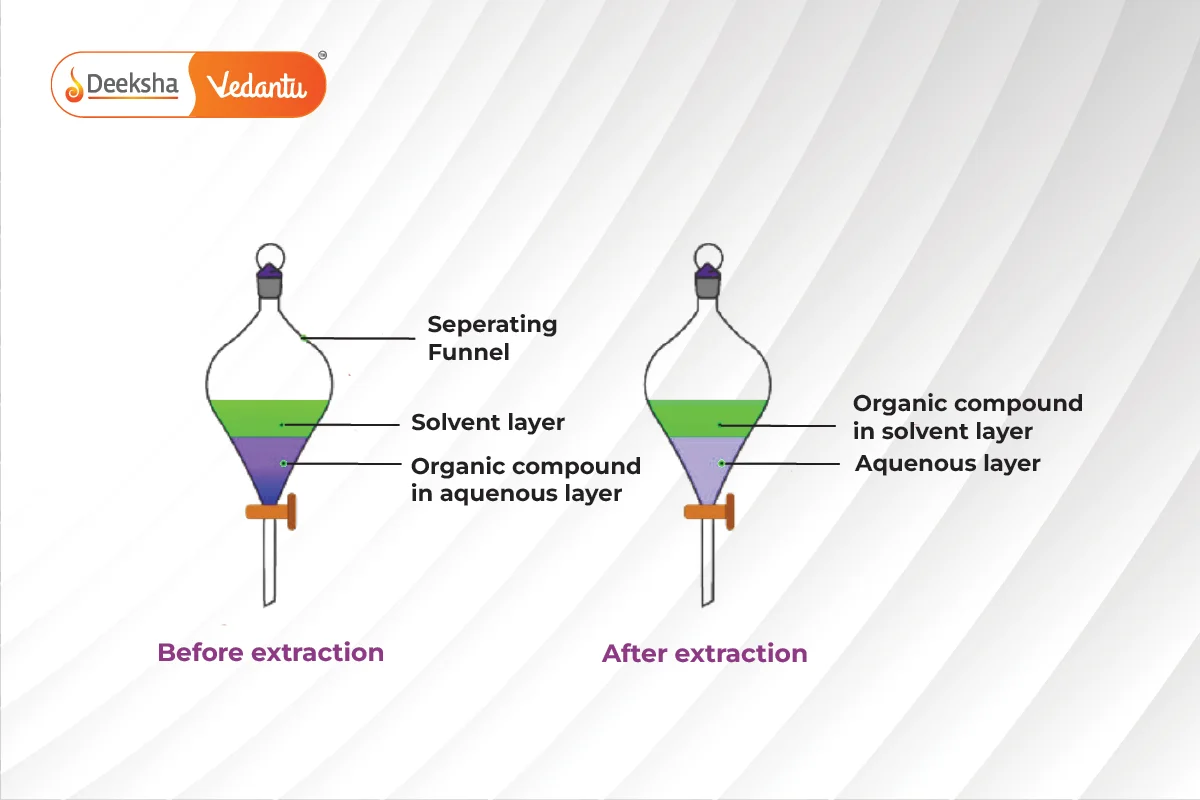
Differential Extraction
Differential extraction is used to separate organic compounds from an aqueous solution using an organic solvent that has higher solubility for the compound and is immiscible with water. The organic solvent forms layers that can be separated using a separating funnel. The compound is then recovered by distillation or evaporation.
Applications of Chromatography
Chromatography is crucial in bioanalytical chemistry for separating, isolating, and purifying proteins from complex mixtures. It is used in various protein purification strategies, including reversed-phase chromatography, ion exchange chromatography, affinity chromatography, and size exclusion chromatography.
FAQs
A chromatogram is a recorded plot showing the separation of components in chromatography based on their retention times.
Chromatography separates, isolates, and purifies proteins from complex mixtures, essential in protein purification strategies.
Differential extraction separates organic compounds from an aqueous solution using an immiscible organic solvent.
The main types are adsorption chromatography, thin layer chromatography, column chromatography, and partition chromatography.
Chromatography is a technique for separating, purifying, and testing compounds by using a stationary phase and a mobile phase.
Related Topics
- Some Important Carbon Compounds – Ethanol And Ethanoic Acid
- Acids, Bases, and Salts
- Bonding In Carbon – The Covalent Bond
- Suspension
- Periodicity of Valence or Oxidation States of Elements
- How Do Metals and Non-Metals React?
- Versatile Nature Of Carbon
- Chemical Formula
- Modern Periodic Table
- Chemistry FAQs
- Chemical Properties Of Carbon Compounds
- Hybridization
- Soaps And Detergents
- Reactivity Series
- Protein Structure And Levels of Protein











Get Social