Acids and bases are common chemicals that react together to form salt and water. The word “acid” comes from the Latin word ‘acere,’ meaning ‘sour.’
In daily life, we use many acids. For example, citric acid is found in orange juice, lactic acid in sour milk, and acetic acid in vinegar. These acids and bases combine to form chemical bonds, helping us understand their properties.
Definitions:
- Acid: A substance that donates a hydrogen ion (H+). It tastes sour and turns blue litmus paper red.
- Base: A substance that accepts a hydrogen ion. It tastes bitter, feels slippery, and turns red litmus paper blue.
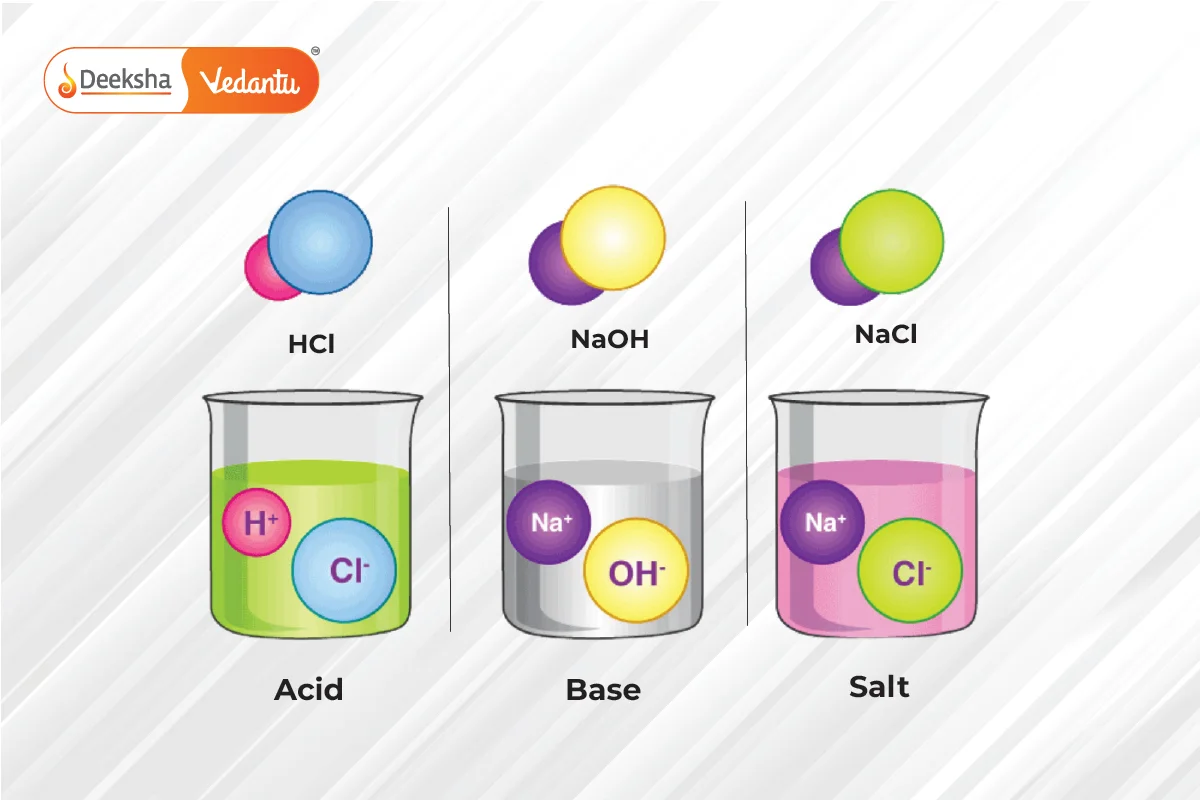
Types of Definitions:
- Arrhenius Definition: Acids produce H+ ions, and bases produce OH- ions in a solution.
- Bronsted-Lowry Definition: Acids donate protons (H+), and bases accept protons.
- Lewis Definition: Acids accept electron pairs, and bases donate electron pairs.
pH Scale:
The pH scale measures how acidic or basic a substance is, ranging from 0 (most acidic) to 14 (most basic). Litmus paper can also be used to test this: blue paper turns red for acids, and red paper turns blue for bases.
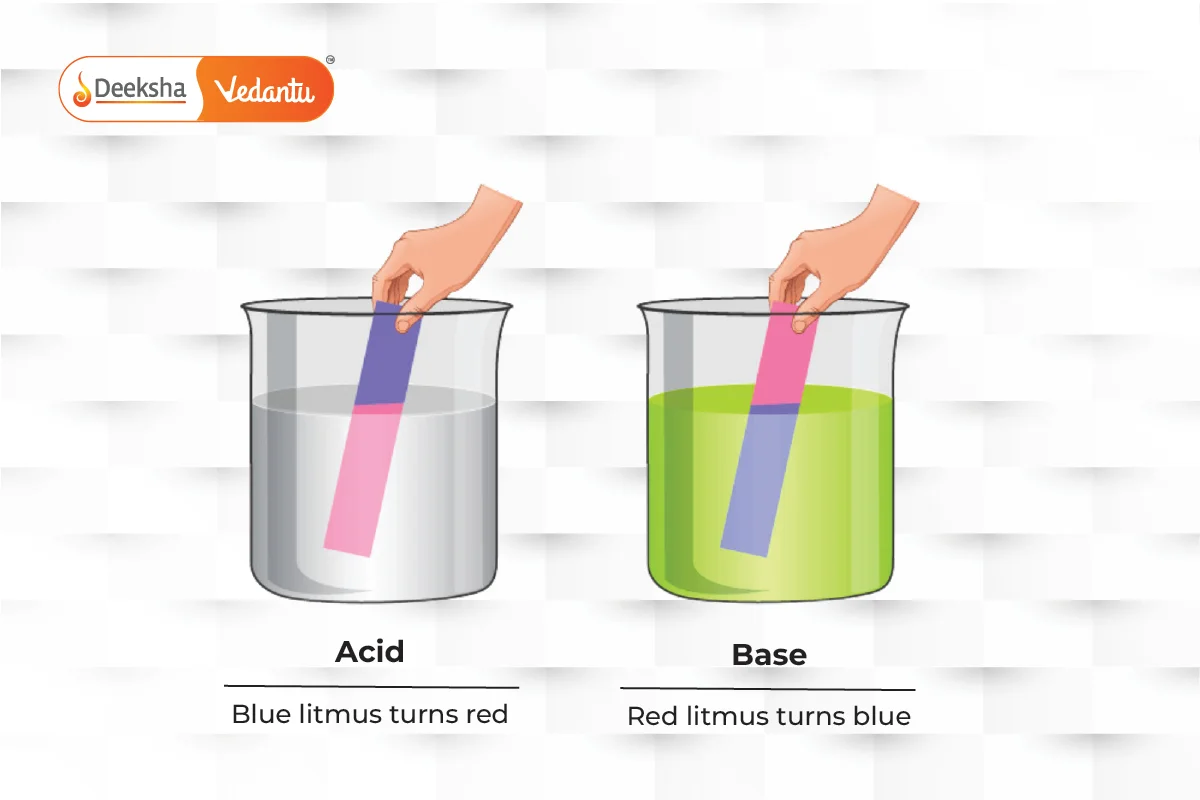
Properties:
Acids:
- Corrosive
- Conduct electricity
- pH less than 7
- Produce hydrogen gas with metals
- Example: Sulfuric acid (H2SO4), Hydrochloric acid (HCl)
Bases:
- Slippery
- Release OH- ions in water
- Conduct electricity
- pH greater than 7
- Example: Sodium hydroxide (NaOH), Calcium hydroxide (Ca(OH)2)
Neutral Substances:
- Neither acidic nor basic
- pH of 7
- No effect on litmus paper
- Example: Water (H2O), Common salt (NaCl)
Differences:
- Acids: Release H+ ions, turn blue litmus red, taste sour, pH 1-7.
- Bases: Release OH- ions, turn red litmus blue, taste bitter, pH 7-14.
Theories:
- Arrhenius Theory: Acids release H+ ions, bases release OH- ions.
- Bronsted-Lowry Theory: Acids are proton donors, bases are proton acceptors.
- Lewis Theory: Acids accept electron pairs, bases donate electron pairs.
Conjugate Acids and Bases:
- Acids and bases form pairs that differ by one proton.
- Example: CH3COOH+H2O↔CH3COO−+H3O+
- Acid: CH3COOH, Conjugate base: CH3COO−
- Base: H2O, Conjugate acid: H3O+
Uses:
Acids:
- Vinegar for food preservation
- Citric acid in lemon/orange juice
- Sulphuric acid in batteries
- Phosphoric acid in soft drinks
Bases:
- Sodium hydroxide in soap making
- Calcium hydroxide in bleaching powder
- Magnesium hydroxide as an antacid
- Ammonium hydroxide in labs
FAQs
Acids are used in food preservation, batteries, and soft drinks. Bases are used in soap making, bleaching powder, and antacids.
Conjugate acids and bases are pairs of substances that differ by one proton. An acid becomes its conjugate base after donating a proton, and a base becomes its conjugate acid after accepting a proton.
Common acids: Citric acid (in citrus fruits), acetic acid (in vinegar), lactic acid (in sour milk).
Common bases: Sodium hydroxide (in soap), calcium hydroxide (in bleaching powder), magnesium hydroxide (in antacids).
The pH scale measures the acidity or basicity of a substance, ranging from 0 (very acidic) to 14 (very basic).
Acids taste sour and turn blue litmus paper red, while bases taste bitter, feel slippery, and turn red litmus paper blue.
Acids are substances that donate hydrogen ions (H+), and bases are substances that accept hydrogen ions.
Related Topics
- 118 Elements – Their Symbols and Atomic Number
- Atomic Mass of Elements
- Chemical Properties Of Carbon Compounds
- Versatile Nature Of Carbon
- Corrosion
- Bonding In Carbon – The Covalent Bond
- Natural Resources
- How Do Metals and Non-Metals React?
- Reactivity Series
- Protein Structure And Levels of Protein
- Occurrence of Metals
- Physical Properties Of Metals And Non-Metals
- Differential Extraction Chromatography
- Types of Chemical Reactions
- How Strong Are Acid Or Base Solutions?











Get Social