Thermodynamics involves the study of heat, temperature, and the conversion between heat and other energy forms. The four laws of thermodynamics describe how these quantities behave and provide quantitative descriptions. The term ‘thermodynamics’ was coined by William Thomson in 1749.
What is Thermodynamics?
Thermodynamics is a branch of physics that focuses on heat, work, temperature, and their relationship to energy, radiation, and the physical properties of matter. It explains how thermal energy (energy from heat) is converted to and from other energy forms and how this process affects matter. Thermal energy comes from the movement of tiny particles within an object—the faster they move, the more heat is produced. Thermodynamics looks at the initial and final states of these energy transformations and is considered a macroscopic science, meaning it deals with large-scale systems rather than individual molecules.
Branches of Thermodynamics
Thermodynamics is divided into four main branches:
- Classical Thermodynamics: This branch uses a macroscopic approach to study the behavior of matter, considering units like temperature and pressure to calculate properties and predict characteristics during processes.
- Statistical Thermodynamics: This branch focuses on the properties and interactions of individual molecules to understand the behavior of a large group of molecules.
- Chemical Thermodynamics: This branch studies the relationship between work and heat in chemical reactions and changes of state.
- Equilibrium Thermodynamics: This branch examines the transformations of energy and matter as they approach equilibrium.
Basic Concepts of Thermodynamics – Thermodynamic Terms
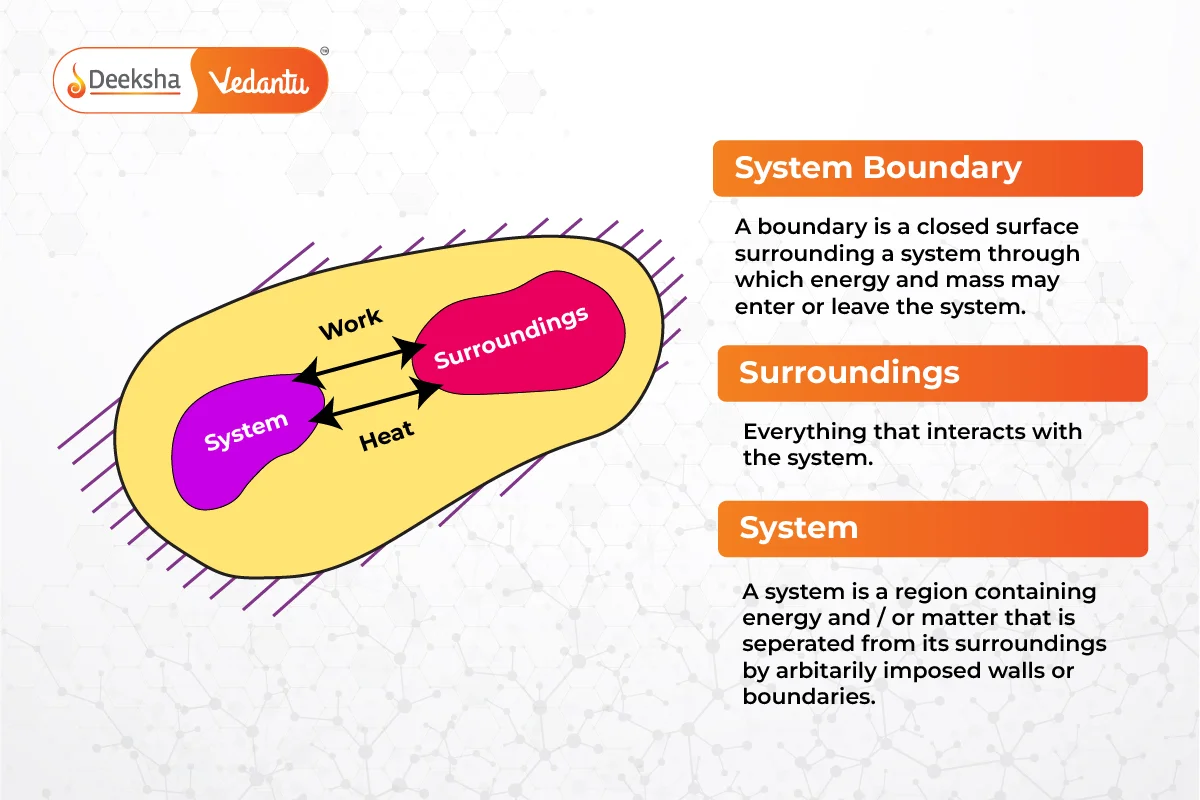
System
A thermodynamic system is a defined quantity of matter with specific boundaries, which can be real or imaginary, fixed or movable. The focus is on the changes and interactions within this system. There are three types of systems:
- Isolated System: No exchange of energy or mass with the surroundings. An example is the universe.
- Closed System: Energy can be transferred across the boundary, but mass cannot. Examples include a refrigerator and gas compression in a piston-cylinder assembly.
- Open System: Both energy and mass can be transferred between the system and surroundings. A steam turbine is an example.
Surrounding
The surrounding is everything outside the system that directly affects its behavior.
Thermodynamic Process
A thermodynamic process involves energetic changes within a system, affecting pressure, volume, and internal energy. The main types are:
- Adiabatic Process: No heat transfer occurs.
- Isochoric Process: Volume remains constant, and no work is done.
- Isobaric Process: Pressure remains constant.
- Isothermal Process: Temperature remains constant.
Thermodynamic Equilibrium
A system is in thermodynamic equilibrium when no properties change over time. This includes:
- Thermal Equilibrium: Uniform temperature throughout the system.
- Mechanical Equilibrium: No pressure changes within the system.
- Chemical Equilibrium: No variation in chemical composition over time.
- Phase Equilibrium: The mass of each phase remains constant in a two-phase system.
A system achieves thermodynamic equilibrium when it is in thermal, mechanical, and chemical equilibrium.
Thermodynamic Properties
These properties define a system’s state and can be classified as intensive or extensive:
- Intensive Properties: Do not depend on the amount of matter (e.g., pressure, temperature).
- Extensive Properties: Depend on the system’s mass (e.g., volume, energy, enthalpy).
What is Enthalpy?
Enthalpy measures the energy in a thermodynamic system. It represents the total heat content of the system, calculated as the sum of the internal energy and the product of the system’s pressure and volume. The mathematical expression for enthalpy (H) is:
H=E+PV
where
E is internal energy, P is pressure, and V is volume.
What is Entropy?
Entropy is a measure of the disorder or randomness in a system. It depends on the system’s physical state or condition. For example, a solid has lower entropy compared to a gas because the particles in a solid are less free to move than those in a gas.
Laws of Thermodynamics
The laws of thermodynamics define fundamental physical quantities such as energy, temperature, and entropy, and describe their behavior in thermodynamic systems at thermal equilibrium. There are four laws:
- Zeroth Law of Thermodynamics: If two bodies are each in thermal equilibrium with a third body, they are also in thermal equilibrium with each other.
- First Law of Thermodynamics: Also known as the law of conservation of energy, it states that energy cannot be created or destroyed, only transformed from one form to another.
- Second Law of Thermodynamics: Entropy in an isolated system always increases. An isolated system naturally evolves towards a state of maximum entropy, or thermal equilibrium.
- Third Law of Thermodynamics: As temperature approaches absolute zero, the entropy of a system approaches a constant minimum. For a pure crystalline substance at absolute zero, the entropy is zero, provided the crystal has only one state with minimum energy.
These laws provide a framework for understanding how energy and matter behave in thermodynamic processes.
The second law dictates that natural processes tend to move towards a state of greater entropy or disorder. It explains why heat flows from hot to cold objects and why certain reactions occur spontaneously while others do not.
The first law of thermodynamics, or conservation of energy, can be seen in many everyday situations, such as heating water on a stove (converting electrical energy to thermal energy) or riding a bicycle (converting chemical energy from food into mechanical energy).
Thermal equilibrium occurs when two systems in contact with each other cease to exchange heat, resulting in the same temperature throughout both systems.
A thermodynamic system is a specific portion of matter or a space chosen for analysis. It is separated from its surroundings by a boundary which can be real or imaginary, fixed or movable.
Entropy is a measure of the disorder or randomness in a system. It quantifies how much energy in a system is unavailable for doing work and tends to increase in isolated systems.
Related Topics
- The Human Eye
- Fleming’s Left-Hand Rule and Right-Hand Rule
- Bernoullis Principle
- Protection Against Earthquake
- Spherical Mirrors
- The Human Eye And The Colourful World
- Refraction Of Light Through A Prism
- Heating Effect Of Electric Current
- Noise Pollution
- Human Eye – Structure and Functioning
- Electric Potential And Potential Difference
- Concave Mirrors and Convex Mirrors
- What is Hypothesis?
- Physics FAQs
- Acceleration






Get Social