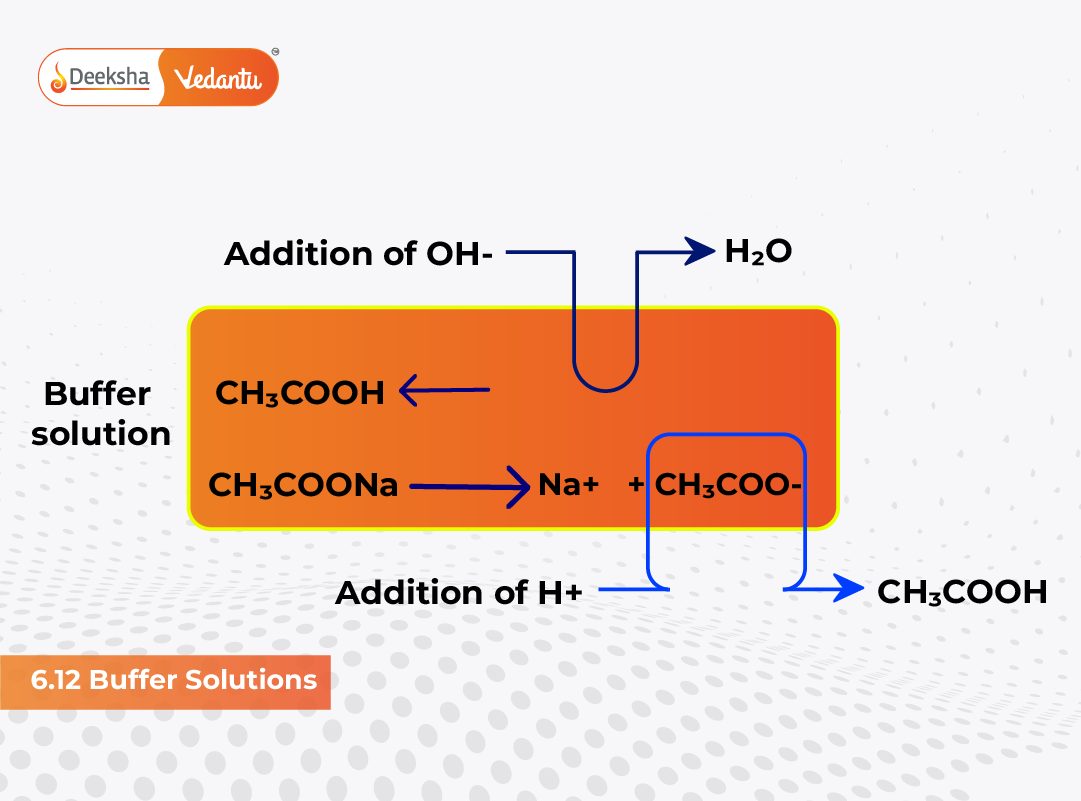
In both living organisms and chemical systems, maintaining a stable pH is essential. Buffer solutions play a key role in achieving this balance. Many body fluids such as blood and urine have fixed pH ranges, and any deviation can indicate serious malfunction. Similarly, numerous biochemical and industrial processes require constant pH for optimal results. In essence, buffer solutions are those that resist changes in pH upon dilution or upon the addition of small amounts of acid or alkali.
Buffer solutions are thus critical in medical formulations, cosmetics, food chemistry, and laboratory experiments. For example, the pH of blood is maintained around 7.4 using buffer systems composed of carbonic acid and bicarbonate ions. Even a slight variation in this pH can cause physiological imbalance.
Designing a Buffer Solution
To design a buffer solution of a specific pH, one must understand the relationship between the acid dissociation constant (Kₐ) or base dissociation constant (Kᵦ) and the ratio of the concentrations of the acid and its conjugate base (or vice versa for a basic buffer). Knowledge of pKₐ, pKᵦ, and equilibrium constants is key in preparing a buffer of known pH.
Preparation of Acidic Buffer
An acidic buffer typically consists of a weak acid and its salt with a strong base. For example, a mixture of acetic acid (CH₃COOH) and sodium acetate (CH₃COONa) acts as an effective buffer near pH 4.75.
When acetic acid is dissolved in water, it partially ionizes:
CH₃COOH ⇌ H⁺ + CH₃COO⁻
If sodium acetate is added, it dissociates completely to release acetate ions (CH₃COO⁻). The common ion (CH₃COO⁻) suppresses the ionization of acetic acid (common ion effect), thus maintaining nearly constant [H⁺] and resisting pH change.
The expression for the equilibrium constant is:
Kₐ = [H⁺][CH₃COO⁻] / [CH₃COOH]
Rearranging:
[H⁺] = Kₐ × [CH₃COOH] / [CH₃COO⁻]
Taking the logarithm of both sides:
pH = pKₐ + log([CH₃COO⁻] / [CH₃COOH])
This equation is known as the Henderson–Hasselbalch equation, which relates the pH of a buffer to its acid and salt concentrations.
If the concentrations of the acid and salt are equal, then pH = pKₐ. Hence, by adjusting their ratio, buffers of desired pH can be prepared.
For example, to make a buffer of pH 4.76, one can use equimolar solutions of acetic acid and sodium acetate. If the ratio of acetate to acetic acid changes, the pH shifts slightly but still resists large fluctuations.
Preparation of Basic Buffer
A basic buffer is made by combining a weak base with its conjugate acid. The classic example is a mixture of ammonium hydroxide (NH₄OH) and ammonium chloride (NH₄Cl).
NH₄OH partially ionizes:
NH₄OH ⇌ NH₄⁺ + OH⁻
Adding ammonium chloride introduces NH₄⁺ ions, which suppress further ionization of NH₄OH, maintaining a constant OH⁻ concentration. The expression for the base equilibrium constant is:
Kᵦ = [NH₄⁺][OH⁻] / [NH₄OH]
Rearranging:
[OH⁻] = Kᵦ × [NH₄OH] / [NH₄⁺]
Taking logarithms gives:
pOH = pKᵦ + log([NH₄⁺] / [NH₄OH])
Using the relation pH + pOH = 14, the buffer pH can be derived as:
pH = 14 – (pKᵦ + log([NH₄⁺] / [NH₄OH]))
Alternatively, it can be expressed as:
pH = pKₐ + log([Base] / [Conjugate acid]), where pKₐ = 14 – pKᵦ.
For ammonium buffer systems where pKᵦ = 4.75, the pH value is approximately 9.25, making it effective for maintaining basic environments.
Properties of Buffer Solutions
- Resists pH change: Buffers minimize drastic changes in pH upon addition of small acid or alkali quantities.
- Constant pH on dilution: The ratio of acid to conjugate base (or vice versa) remains nearly constant even when diluted.
- Specific pH range: Each buffer works best within ±1 pH unit of its pKₐ or pKᵦ.
- Reversible equilibrium: Buffers establish a dynamic equilibrium between weak acid/base and its conjugate species.
Example Calculation
For a buffer made from 0.1 M acetic acid and 0.1 M sodium acetate:
pH = pKₐ + log([Salt] / [Acid])
= 4.76 + log(1) = 4.76
If sodium acetate concentration doubles, pH becomes:
pH = 4.76 + log(2) = 5.06
This shows that pH changes only slightly despite the variation in concentration, confirming buffer stability.
Importance of Buffer Solutions
- Biological significance: Maintains pH in body fluids, e.g., blood (pH ≈ 7.4) and intracellular fluids.
- Industrial use: Controls pH in fermentation, pharmaceuticals, and textile processing.
- Analytical chemistry: Essential in titrations and maintaining constant pH during reactions.
- Environmental relevance: Regulates pH in natural waters, preventing acidification.
- Cosmetic and medical applications: Ensures stability in creams, lotions, and injectable drugs.
Key Buffer Equations Recap
- For acidic buffer: pH = pKₐ + log([Salt] / [Acid])
- For basic buffer: pH = 14 – (pKᵦ + log([Salt] / [Base]))
- Special case (NH₄Cl–NH₄OH): pH = 9.25 + log([NH₄⁺] / [NH₄OH])
The logarithmic term remains constant during dilution, ensuring the pH of a buffer solution remains nearly unchanged.
Conclusion
Buffer solutions are essential in both natural and laboratory systems where maintaining a constant pH is critical. They provide chemical stability, facilitate biological functioning, and support analytical precision. Understanding the chemistry behind buffers – including the Henderson–Hasselbalch equation, buffer capacity, and the relationship between conjugate acid-base pairs – equips NEET and JEE aspirants with foundational knowledge for solving equilibrium-based questions.





Get Social