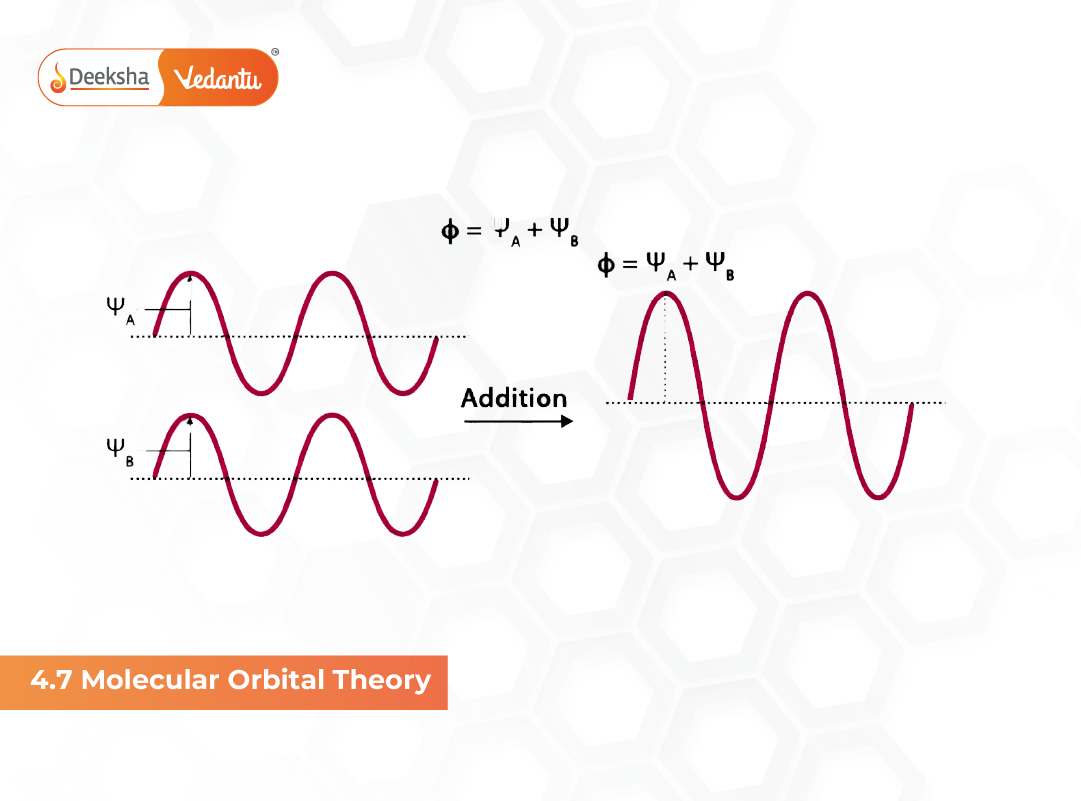
Molecular Orbital Theory (MOT), introduced by F. Hund and R.S. Mulliken in 1932, explains bonding in molecules using molecular orbitals formed from atomic orbitals. Unlike Valence Bond Theory, MOT describes electrons as delocalised across the entire molecule. This helps explain magnetic properties, bond order, stability, molecular geometry and existence of certain molecules that VBT cannot properly justify. MOT is extremely important for NCERT, NEET and JEE because it connects quantum mechanics with real molecular behaviour.
Salient Features of Molecular Orbital Theory
Key features of MOT:
- Electrons in molecules occupy molecular orbitals similar to atomic orbitals in atoms.
- Molecular orbitals are formed by linear combination of atomic orbitals (LCAO) with comparable energy and proper symmetry.
- Electrons in atomic orbitals are influenced by one nucleus, while electrons in molecular orbitals are influenced by two or more nuclei.
- Number of molecular orbitals formed = number of combining atomic orbitals.
- Molecular orbitals are classified as bonding and antibonding.
- Bonding orbitals have lower energy and high stability, while antibonding orbitals have higher energy and lower stability.
- Electrons fill molecular orbitals according to Aufbau Principle, Pauli’s Exclusion Principle and Hund’s Rule.
- MOT helps explain bond order, magnetism, stability, molecular geometry and energy distribution.
Formation of Molecular Orbitals: Linear Combination of Atomic Orbitals (LCAO)
The LCAO method states that wave functions of atomic orbitals combine to form molecular orbitals:
- ψ (MO) = ψₐ + ψᵦ → Bonding molecular orbital (σ or π)
- ψ (MO) = ψₐ – ψᵦ → Antibonding molecular orbital (σ or π)**
Conditions for effective combination:
- Comparable energy levels
- Proper symmetry orientation
- Significant orbital overlap
Types of Molecular Orbitals
Based on overlap type:
- σ (sigma) → Axial overlap
- π (pi) → Lateral overlap
- σ* and π* → Antibonding counterparts
Orbital Promotion & Hybridisation (PCl₅, SF₆, CH₄)
Before hybridisation, atoms undergo orbital promotion to increase unpaired electrons for bonding.
Example – CH₄ (Methane):
Carbon (atomic state): 1s² 2s² 2p² → Only 2 unpaired electrons
Promotion: 2s → 2p → 1s² 2s¹ 2p³ → 4 unpaired electrons
Hybridisation: sp³ → Tetrahedral geometry (109.5°)
Example – PCl₅ (Phosphorus Pentachloride):
Phosphorus: 3s² 3p³ → after promotion → 3s¹ 3p³ 3d¹ → sp³d → Trigonal bipyramidal
Example – SF₆ (Sulfur Hexafluoride):
Sulfur: 3s² 3p⁴ → 3s¹ 3p³ 3d² → sp³d² → Octahedral geometry
Equatorial & Axial Bonds (in Trigonal Bipyramidal PCl₅)
- Equatorial Bonds → Lie in a plane (120° apart)
- Axial Bonds → Perpendicular to plane (180° apart)
- Axial bonds experience more repulsion → longer and weaker
- Equatorial bonds are more stable
Understanding this helps in predicting chemical reactivity of PCl₅.
Hybridisation Type Table
| Molecule | Hybridisation | Shape | Example |
| sp | Linear | BeCl₂, C≡C-H | |
| sp² | Trigonal planar | BF₃, C₂H₄ | |
| sp³ | Tetrahedral | CH₄ | |
| sp³d | Trigonal bipyramidal | PCl₅ | |
| sp³d² | Octahedral | SF₆ |
Sigma (σ) & Pi (π) Bonds – Ethene & Ethyne
Ethene (C₂H₄):
- Carbon is sp² hybridised
- 3 sp² orbitals form σ bonds (2 with H, 1 with C)
- 1 unhybridised p orbital forms π bond → C=C double bond
Bonding: 1 σ + 1 π
Ethyne (C₂H₂):
- Carbon is sp hybridised
- 2 sp orbitals form σ bonds (1 with H, 1 with C)
- 2 p orbitals form two π bonds → C≡C triple bond
Bonding: 1 σ + 2 π
Sigma bonds allow rotation, while pi bonds restrict rotation → causes rigidity in double & triple bonded molecules.
Energy Level Diagrams of Molecular Orbitals
For diatomic molecules (first-row elements):
σ1s < σ1s < σ2s < σ2s < (π2px = π2py) < σ2pz < (π2px = π2py) < σ*2pz
For B₂, C₂, N₂ (experimental modification):
σ1s < σ1s < σ2s < σ2s < (π2px = π2py) < σ2pz < π2px = π2py < σ*2pz
Electronic Configuration and Molecular Behaviour
Bond Order
Bond order = ½ (bonding electrons – antibonding electrons)
- Bond order > 0 → stable
- Bond order = 0 → does not exist
Higher bond order = stronger bond & shorter bond length
Magnetic Nature
- Unpaired electrons → Paramagnetic
- All electrons paired → Diamagnetic
Bonding in Some Homonuclear Diatomic Molecules
| Molecule | Bond Order | Nature | Magnetic Behaviour |
| H₂ | 1 | Stable | Diamagnetic |
| He₂ | 0 | Does not exist | — |
| N₂ | 3 | Stable | Diamagnetic |
| O₂ | 2 | Stable | Paramagnetic |
Example – H₂:
- MO formed: σ1s & σ*1s
- Bond order = 1 → Stable molecule
Example – O₂:
- Bond order = 2
- Two unpaired electrons → Paramagnetic
MCQ Practice Questions
- Which principle is used to explain electron filling in molecular orbitals according to MOT?
a) Heisenberg’s Principle b) Aufbau Principle c) Dalton’s Law d) Boyle’s Law - In PCl₅, how many equatorial bonds are present?
a) 2 b) 3 c) 4 d) 5 - Ethyne contains how many π bonds?
a) 0 b) 1 c) 2 d) 3 - What is the bond order of O₂ molecule?
a) 1 b) 1.5 c) 2 d) 3 - Unpaired electrons in molecular orbitals lead to:
a) Diamagnetism b) Paramagnetism c) No effect d) Stability - The hybridisation of carbon in ethene (C₂H₄) is:
a) sp b) sp² c) sp³ d) sp³d - Orbital promotion occurs to:
a) Decrease bond order b) Increase unpaired electrons c) Lower energy d) Remove electrons - Axial bonds in PCl₅ are:
a) Shorter and stronger b) Longer and weaker c) Same as equatorial d) Absent - The molecular orbital formed by lateral overlap is:
a) σ b) σ* c) π d) s - Bond order = ½ (bonding electrons – antibonding electrons). What is bond order if bonding e⁻ = 8 and antibonding e⁻ = 4?
a) 1 b) 2 c) 3 d) 4
Answers: 1-b, 2-b, 3-c, 4-c, 5-b, 6-b, 7-b, 8-b, 9-c, 10-b
FAQs
Q1: What is molecular orbital theory?
It explains bonding using delocalised molecular orbitals formed from atomic orbitals.
Q2: Why is O₂ paramagnetic?
Because its MO configuration has two unpaired electrons in π* orbitals.
Q3: What is orbital promotion?
It is the process where electrons move to higher orbitals to increase unpaired electrons before bonding.
Q4: What is the difference between sigma and pi bonds?
Sigma bonds are formed by axial overlap and allow rotation, while pi bonds are lateral overlaps and restrict rotation.
Q5: What are equatorial and axial bonds?
In PCl₅, equatorial bonds lie in one plane while axial bonds are perpendicular to it. Axial bonds are longer and weaker.
Conclusion
Molecular Orbital Theory gives a complete quantum-based explanation of bonding, geometry, magnetism and stability of molecules. It supports hybridisation, sigma–pi bonding, orbital promotion and bond order analysis, making it extremely important for NCERT, NEET and JEE students. Understanding MOT along with hybridisation tables, axial–equatorial bonds and sigma–pi bonds builds strong conceptual clarity for exams.





Get Social