Alkenes are an important class of unsaturated hydrocarbons that contain at least one carbon–carbon double bond (C=C). This double bond introduces a level of reactivity and structural behaviour far different from alkanes. Represented by the general formula CₙH₂ₙ, alkenes form the basis of many naturally occurring and industrially significant compounds. Their distinctive features such as restricted rotation around the double bond, geometrical isomerism, and electrophilic addition reactions make them central to understanding organic chemistry.
Alkenes occur widely in nature, including plant oils and natural products. They are also produced industrially in petroleum cracking processes and serve as key precursors for polymers, alcohols, and various synthetic intermediates.
Structure of Double Bond
The carbon–carbon double bond in alkenes consists of two different types of covalent bonds:
- One sigma (σ) bond, resulting from the head-on overlap of sp² hybrid orbitals.
- One pi (π) bond, formed by the lateral or sideways overlap of unhybridised p-orbitals.
Geometry and Hybridisation
Each carbon atom involved in the double bond is sp² hybridised, resulting in three sigma bonds arranged in a trigonal planar geometry with bond angles of approximately 120°. The unhybridised p-orbitals overlap sideways to form the π bond.
This π bond is weaker than the σ bond and lies above and below the plane of the molecule. Because of its electron density, the π bond acts as an electrophilic attack site during reactions.
Restricted Rotation
Unlike alkanes, alkenes do not have free rotation around the C=C bond. The π bond restricts rotation because breaking it would require considerable energy. This restriction gives rise to geometrical isomerism, a key characteristic of alkenes.
Nomenclature
Alkenes are named using the IUPAC system, where the suffix “-ene” denotes the presence of a double bond.
Rules for Naming Alkenes
- Identify the longest carbon chain containing the double bond.
- Number the carbon chain beginning from the end closer to the double bond.
- Indicate the position of the double bond using the lowest possible number.
- Identify and name substituents, placing them in alphabetical order.
- Use prefixes such as di-, tri-, or tetra- if more than one double bond is present.
Examples
- CH₂=CH₂ → Ethene
- CH₂=CH-CH₃ → Propene
- CH₃-CH=CH-CH₃ → But-2-ene
- CH₂=C(CH₃)-CH₃ → 2-Methylprop-1-ene
These naming rules ensure clarity and uniformity across organic molecules.
Isomerism in Alkenes
Alkenes exhibit multiple types of isomerism, each reflecting the structural diversity enabled by the presence of the double bond.
1. Structural Isomerism
This arises when alkenes differ in the arrangement of the carbon chain or the position of the double bond.
Chain Isomerism
Occurs when carbon skeletons vary:
- Example: But-1-ene and 2-methylpropene
Position Isomerism
Occurs when the double bond is located at different positions along the carbon chain:
- Example: Pent-1-ene and Pent-2-ene
2. Geometrical Isomerism (Cis-Trans)
Because rotation around the C=C bond is not possible, alkenes with different spatial arrangements of substituents around the double bond form stereoisomers.
- Cis Isomer: Similar groups are on the same side.
- Trans Isomer: Similar groups are on opposite sides.
For instance, But-2-ene exists as cis-but-2-ene and trans-but-2-ene. These isomers show distinct physical properties such as boiling points, melting points, and polarity.
Geometrical isomerism occurs only when each carbon of the double bond has two different substituents.
Preparation of Alkenes
Alkenes can be prepared through several important laboratory and industrial methods. NCERT highlights the following routes:
1. Dehydrohalogenation of Alkyl Halides
Alkyl halides heated with alcoholic KOH undergo β-elimination to form alkenes. The reaction removes HX from adjacent carbon atoms.
Example:
R-CH₂-CH₂-X + KOH (alc.) → R-CH=CH₂ + KX + H₂O
Mechanism:
- Follows the E2 pathway where base abstracts a proton while the leaving group departs.
2. Dehydration of Alcohols
Alcohols when heated with concentrated sulphuric acid or passed over heated alumina undergo dehydration, losing a water molecule to form an alkene.
Conditions:
- Ethanol → 443 K (conc. H₂SO₄)
- Propan-2-ol → 503 K (conc. H₂SO₄)
3. Partial Hydrogenation of Alkynes
Alkynes can be converted to alkenes by using a partially deactivated catalyst such as Lindlar catalyst, which prevents complete hydrogenation.
Example:
R-C≡C-R + H₂ → R-CH=CH-R (over Lindlar catalyst)
4. Cracking of Alkanes
Large alkanes undergo pyrolysis (cracking) to produce smaller alkanes and alkenes. This is widely used in petroleum refineries to produce ethene and propene.
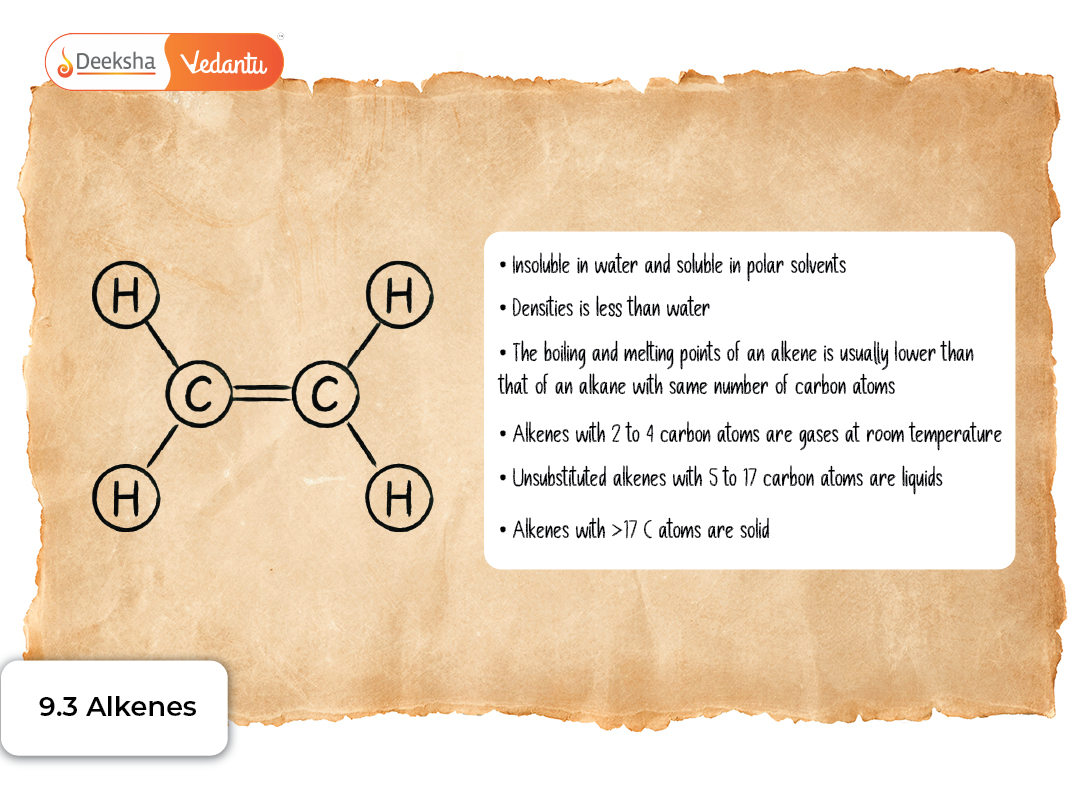
Physical Properties
Alkenes exhibit characteristic physical behaviour:
State and Appearance
- Lower alkenes (ethene, propene, butene) are gases.
- Intermediate alkenes are liquids.
- Higher alkenes are waxy solids.
Solubility
- Insoluble in water (non-polar)
- Soluble in organic solvents
Boiling and Melting Points
- Increase with molecular mass.
- Cis isomers have slightly higher boiling points due to polarity.
Density
- Alkenes are lighter than water and float on its surface.
Chemical Properties and Reactions
Alkenes undergo a wide variety of reactions due to the presence of the electron-rich π bond. This π bond acts as a nucleophile and readily participates in addition reactions, oxidation reactions, and polymerisation. Understanding these reactions is essential for predicting the behaviour of alkenes in organic synthesis.
1. Electrophilic Addition Reactions
Electrophilic addition is the most characteristic reaction of alkenes. These reactions proceed in two fundamental steps:
- Step 1: The electrophile attacks the π bond, forming a carbocation.
- Step 2: The nucleophile attacks the carbocation to form the final product.
This mechanism is widely applicable in hydration, hydrohalogenation, halogenation, and hydrogenation reactions.
Hydrogenation
Hydrogenation is the addition of hydrogen to alkenes in the presence of metal catalysts like Ni, Pt, or Pd. This process breaks the π bond and adds hydrogen atoms to form alkanes.
Features:
- Used in food industries for hydrogenating oils (e.g., margarine production).
- Important in converting unsaturated hydrocarbons into saturated ones.
- Reaction is highly exothermic.
Halogenation
Alkenes react with halogens (Cl₂, Br₂) to form vicinal dihalides.
Key points:
- The reaction is fast and produces colourless products.
- Decolourisation of bromine water is used as a qualitative test for unsaturation.
- Occurs via a cyclic halonium ion intermediate instead of a free carbocation, ensuring anti-addition.
Hydrohalogenation
Hydrohalogenation involves the addition of hydrogen halides (HCl, HBr, HI).
Important points:
- Follows Markovnikov’s rule in the absence of peroxides.
- Forms more substituted haloalkanes.
- Reaction rate increases from HI > HBr > HCl.
Mechanistic Insight: Hydrohalogenation proceeds via a carbocation intermediate, therefore the stability of the carbocation determines the major product.
2. Markovnikov’s Rule
Markovnikov’s rule states:
“During the addition of HX to an unsymmetrical alkene, the hydrogen atom attaches itself to the carbon atom already carrying more hydrogen atoms.”
Reason:
- Formation of the more stable carbocation intermediate.
- Tertiary carbocations > secondary > primary in stability.
This regioselectivity is extremely important in predicting the final product.
3. Anti-Markovnikov Addition
This addition occurs only for HBr in the presence of organic peroxides.
Key points:
- Follows a free radical mechanism.
- Bromine radical adds to the less substituted carbon.
- Hydrogen attaches in the next step.
This effect is called the Kharasch effect or the Peroxide effect.
4. Oxidation Reactions
Alkenes undergo oxidation reactions depending on the oxidising agent used.
Cold, Dilute KMnO₄ (Baeyer’s Reagent)
- Converts alkenes into vicinal diols.
- The solution turns from purple to colourless.
- Used as a test for unsaturation.
Hot, Concentrated KMnO₄
- Causes oxidative cleavage of the double bond.
- Produces aldehydes, ketones, or acids depending on the substituents.
Example: R–CH=CH–R → R–COOH + R–COOH (after oxidative cleavage)
5. Ozonolysis
Ozone adds to alkenes to form ozonides, which upon hydrolysis yield carbonyl compounds.
Products after hydrolysis:
- Aldehydes
- Ketones
Ozonolysis is valuable for detecting the position of double bonds in unknown alkenes.
6. Polymerisation
Alkenes undergo additional polymerisation to give long-chain polymers.
Examples:
- Ethene → Polyethylene
- Propene → Polypropylene
Important Details:
- Polymerisation requires catalysts, heat, or pressure.
- Ziegler–Natta catalysts improve control over polymer structure.
- Resulting polymers have a wide range of applications in packaging, construction, and daily-use products.
JEE/NEET Practice Questions
- JEE Level – Electrophilic Addition (Mechanism-Based)
When HBr is added to propene in the presence of organic peroxides, the major product formed is:
(a) 1-bromopropane
(b) 2-bromopropane
(c) 3-bromopropane
(d) Allyl bromide
Answer: (a) 1-bromopropane - NEET Level – Oxidation Reaction
Cold, dilute KMnO₄ converts alkenes into:
(a) Carboxylic acids
(b) Ketones
(c) Vicinal diols
(d) Aldehydes
Answer: (c) Vicinal diols - JEE Level – Carbocation Stability
Which intermediate is responsible for Markovnikov addition of HX to alkenes?
(a) Carbanion
(b) Free radical
(c) Carbocation
(d) Carbine
Answer: (c) Carbocation - NEET Level – Geometrical Isomerism
Cis–trans isomerism is shown by:
(a) Propene
(b) Ethene
(c) 2-butene
(d) Methene
Answer: (c) 2-butene - JEE Level – Ozonolysis
Ozonolysis of 2-methylprop-1-ene gives:
(a) Formaldehyde + acetone
(b) Two molecules of ethanal
(c) Acetaldehyde + acetone
(d) Propanone only
Answer: (a) Formaldehyde + acetone
Additional Deep-Dive Expansion
Alkenes play a central role in organic synthesis because the double bond can be transformed into a wide variety of functional groups. Their reactivity is dominated by the electrophilic addition mechanism, where the π electrons act as a nucleophile. This makes alkenes extremely valuable in forming new C–C and C–X bonds.
Industrial Importance
- Ethene is the largest-volume organic chemical produced globally.
- Used to manufacture polyethylene, one of the most widely used plastics.
- Propene is the precursor of polypropylene, ABS plastics, and acrylic fibres.
- Alkenes also serve as intermediates in producing alcohols via hydration, aldehydes via hydroformylation, and glycols via oxidation.
Stability of Alkenes
Alkene stability increases with substitution:
Tet > Tri > Di > Mono-substituted.
This is due to hyperconjugation and inductive effects, which stabilise the double bond.
Electrophilic Addition – Advanced Insights
Electrophilic addition reactions rely on the formation of a carbocation intermediate. The more stable this intermediate, the more likely the reaction proceeds in that direction. Hence, Markovnikov’s rule is simply an outcome of carbocation stability.
In the peroxide effect (anti-Markovnikov addition), the mechanism proceeds through free radicals instead of carbocations, reversing regioselectivity.
Oxidative Cleavage – A Tool for Structure Determination
Ozonolysis is often used to determine the position of double bonds in unknown compounds. By analysing the aldehydes or ketones formed on hydrolysis, one can reconstruct the original alkene.
Polymerisation of Alkenes
Alkenes undergo additional polymerisation to form long-chain macromolecules. This process is crucial for the plastics industry:
- Polyethylene → from ethene
- Polypropylene → from propene
- PVC → from chloroethene
- Teflon → from tetrafluoroethene
Catalysts such as Ziegler–Natta and metallocenes allow controlled polymer growth, giving polymers with specific properties.
FAQs
Q1. Why do alkenes show geometrical isomerism?
Because restricted rotation around the double bond prevents interchange of substituent positions.
Q2. What determines the position number in alkenes?
The numbering starts from the end nearer to the double bond.
Q3. Why are alkenes more reactive than alkanes?
The π bond in alkenes is weaker and more exposed.
Q4. What catalyst is used for partial hydrogenation of alkynes?
Lindlar catalyst.
Q5. What is formed during ozonolysis of alkenes?
Aldehydes and ketones depending on substituents.
Conclusion
Alkenes are among the most versatile unsaturated hydrocarbons, forming the foundation of modern industrial chemistry. Their double bond not only influences their geometry but also drives most of their chemical behaviour. From electrophilic addition and oxidation to polymerisation and industrial synthesis, alkenes remain at the core of organic chemistry. At Deeksha Vedantu, we help students master these concepts deeply so they gain confidence for both board exams and competitive exams like NEET and JEE.





Get Social