The strength of an acid or base refers to how easily they dissociate into their respective ions in water. This dissociation determines whether an acid or base is strong or weak. The concentration of hydrogen ions () in acids and hydroxide ions (
) in bases is used to measure their strength.
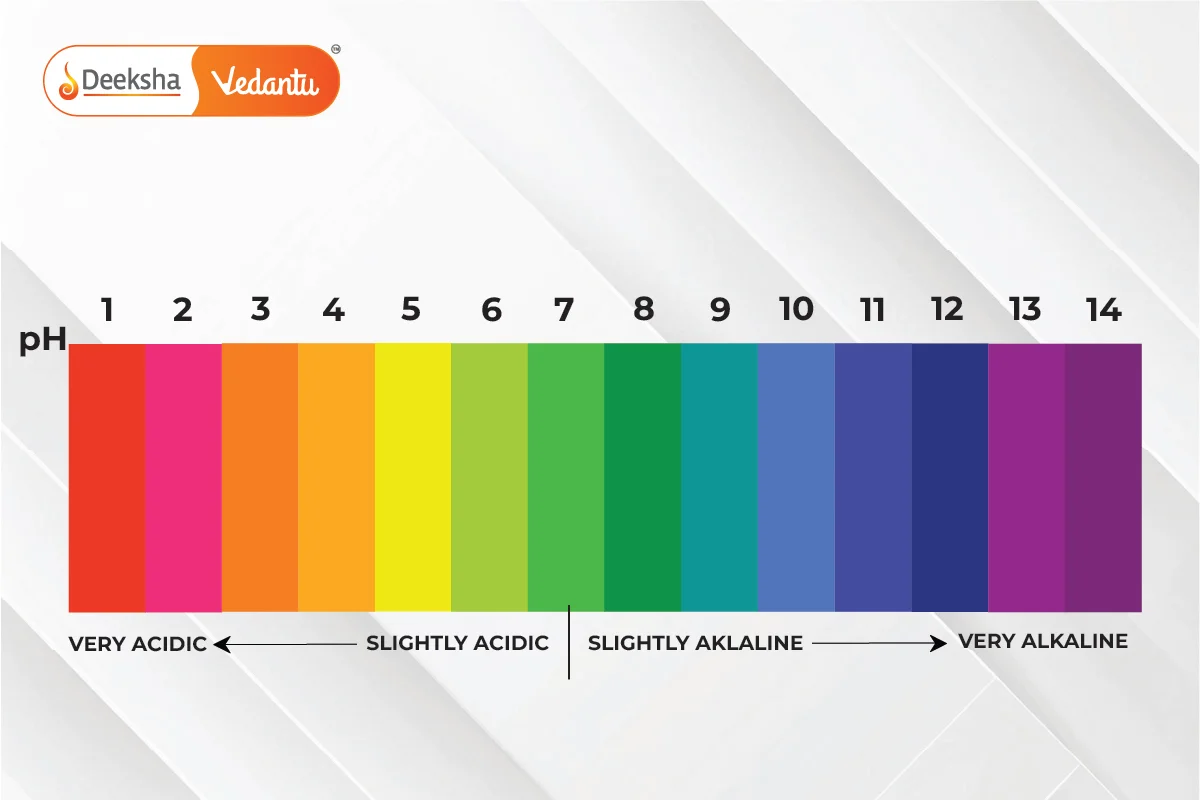
To quantify the strength of acids and bases, we use a scale known as the pH scale. The pH scale measures the concentration of hydrogen ions in a solution and ranges from 0 to 14, with values below 7 indicating acidic solutions, values above 7 indicating basic solutions, and 7 being neutral.
The pH Scale: A Measure of Acidity and Basicity
The pH scale is a logarithmic scale used to specify the acidity or basicity (alkalinity) of a solution. It ranges from 0 (most acidic) to 14 (most basic), with 7 being neutral (neither acidic nor basic, like pure water).
- pH < 7: Acidic solutions.
- pH > 7: Basic (alkaline) solutions.
- pH = 7: Neutral solutions.
Understanding pH
pH is a measure of the concentration of hydrogen ions () in a solution. The lower the pH, the higher the concentration of
ions, and the stronger the acid. Conversely, the higher the pH, the lower the concentration of
ions, and the solution becomes more basic.
Each unit change in pH represents a tenfold change in ion concentration. For example, a solution with a pH of 4 has ten times more hydrogen ions () than a solution with a pH of 5.
Examples of pH Values:
| Substance | pH |
| Hydrochloric acid ( | ~1 |
| Lemon juice | ~2.5 |
| Vinegar (acetic acid) | ~3 |
| Pure water | ~7 |
| Baking soda ( | ~9 |
| Soap solution | ~10 |
| Sodium hydroxide ( | ~13-14 |
- Strong acids like hydrochloric acid (
) have pH values close to 0.
- Weak acids like acetic acid (vinegar) have pH values around 3-5.
- Strong bases like sodium hydroxide (
) have pH values near 14.
- Weak bases like ammonia have pH values around 9-10.
Strong and Weak Acids and Bases
Strong Acids
Strong acids dissociate completely in water, releasing a high concentration of hydrogen ions (). Because of this, they have low pH values (close to 0).
Examples of Strong Acids:
- Hydrochloric acid (
): Used in stomach acid and industrial cleaning.
- Sulfuric acid (
): Used in car batteries and the manufacture of fertilizers.
Dissociation of a Strong Acid:
In this equation, hydrochloric acid completely dissociates into hydrogen ions and chloride ions.
Weak Acids
Weak acids do not dissociate completely in water. They release fewer hydrogen ions (), resulting in higher pH values (typically between 3-6).
Examples of Weak Acids:
- Acetic acid (
): Found in vinegar.
- Citric acid: Found in citrus fruits.
Dissociation of a Weak Acid:
Acetic acid only partially dissociates, with most of the acid remaining in its undissociated form.
Strong Bases
Strong bases dissociate completely in water to release hydroxide ions (), leading to high pH values (near 14).
Examples of Strong Bases:
- Sodium hydroxide (
): Used in drain cleaners and soap making.
- Potassium hydroxide (
): Used in batteries and detergents.
Dissociation of a Strong Base:
Sodium hydroxide completely dissociates into sodium ions and hydroxide ions.
Weak Bases
Weak bases do not dissociate completely in water. They release fewer hydroxide ions (), resulting in lower pH values (typically between 8-10).
Examples of Weak Bases:
- Ammonium hydroxide (
): Used in household cleaning products.
- Ammonia (
): Used in fertilizers and refrigerants.
Dissociation of a Weak Base:
Ammonia reacts with water to form ammonium ions and hydroxide ions, but it only partially dissociates.
Universal Indicator and pH Paper
Universal Indicator:
A universal indicator is a mixture of several indicators that changes color at different pH levels, allowing us to determine the approximate pH of a solution.
- Acidic solutions: Turn the universal indicator from red (strong acid) to orange/yellow (weak acid).
- Neutral solutions: Turn green.
- Basic solutions: Turn blue (weak base) to violet/purple (strong base).
pH Paper:
pH paper is a special kind of indicator that changes color when dipped into an acidic or basic solution, giving a rough estimate of the pH value.
- Color changes: pH paper turns red in acidic solutions and blue in basic solutions.
Applications of pH in Everyday Life
pH in Soil for Plant Growth
Plants require soils of specific pH levels to grow properly.
- Acidic soils (pH below 6.5) can prevent the absorption of essential nutrients.
- Farmers often use lime (calcium hydroxide) to neutralize acidic soils.
pH in the Human Body
The human body maintains a pH of around 7.35-7.45 in the blood, which is essential for proper functioning.
- Stomach acid (
) has a pH around 1-2, aiding in the digestion of food.
- Antacids are used to neutralize excess stomach acid, helping to relieve indigestion.
pH in Water Bodies
Aquatic life depends on the pH of water for survival.
- Most aquatic organisms thrive in water with a pH of 6.5 to 8.5.
- Acid rain (pH below 5.6) can lower the pH of lakes and rivers, harming aquatic life.
pH in Food Preservation
Vinegar (acetic acid) and citric acid are used as preservatives in food to inhibit the growth of bacteria, as bacteria cannot survive in highly acidic conditions.
Practice Questions with Answers
Q1. What is the pH of lemon juice, and why?
- Answer: Lemon juice has a pH of about 2.5 because it contains citric acid, which releases hydrogen ions (
) in solution, making it highly acidic.
Q2. Why is the pH of a strong base higher than that of a weak base?
- Answer: A strong base dissociates completely in water, releasing a higher concentration of hydroxide ions (
), leading to a higher pH value. Weak bases only partially dissociate, releasing fewer hydroxide ions.
Q3. What happens when the pH of a water body falls below 5 due to acid rain?
- Answer: When the pH of a water body falls below 5, the water becomes too acidic for aquatic life, leading to harmful effects on fish and other organisms, and in some cases, can lead to the death of aquatic species.
Q4. How can you measure the strength of an acid or base solution?
- Answer: The strength of an acid or base can be measured using a pH meter, pH paper, or a universal indicator, all of which determine the pH level of the solution.
Q5. What is the significance of pH in the human body?
- Answer: The pH of blood (around 7.35 to 7.45) is essential for various bodily functions, including enzyme activity and oxygen transport. Any deviation from this pH range can lead to health issues.
FAQs
Soil pH affects the availability of nutrients. If the pH is too acidic or too alkaline, plants may not be able to absorb the nutrients they need to grow.
Pure water has a pH of 7, which is neutral.
A universal indicator changes color depending on the pH of the solution, providing a visual way to determine whether the solution is acidic, neutral, or basic.
Strong acids have a pH close to 0 (e.g., hydrochloric acid).
The pH scale measures the concentration of hydrogen ions () in a solution, determining whether the solution is acidic, neutral, or basic.






Get Social