Chemical reactions involve the transformation of substances through breaking and forming of bonds. Among all reaction types, redox reactions-short for reduction-oxidation reactions-play a central role in chemistry. These reactions are responsible for phenomena ranging from rusting of iron and corrosion of metals to respiration, photosynthesis, and combustion. In essence, a redox reaction involves the transfer of electrons between reacting species.
Introduction to Redox Reactions
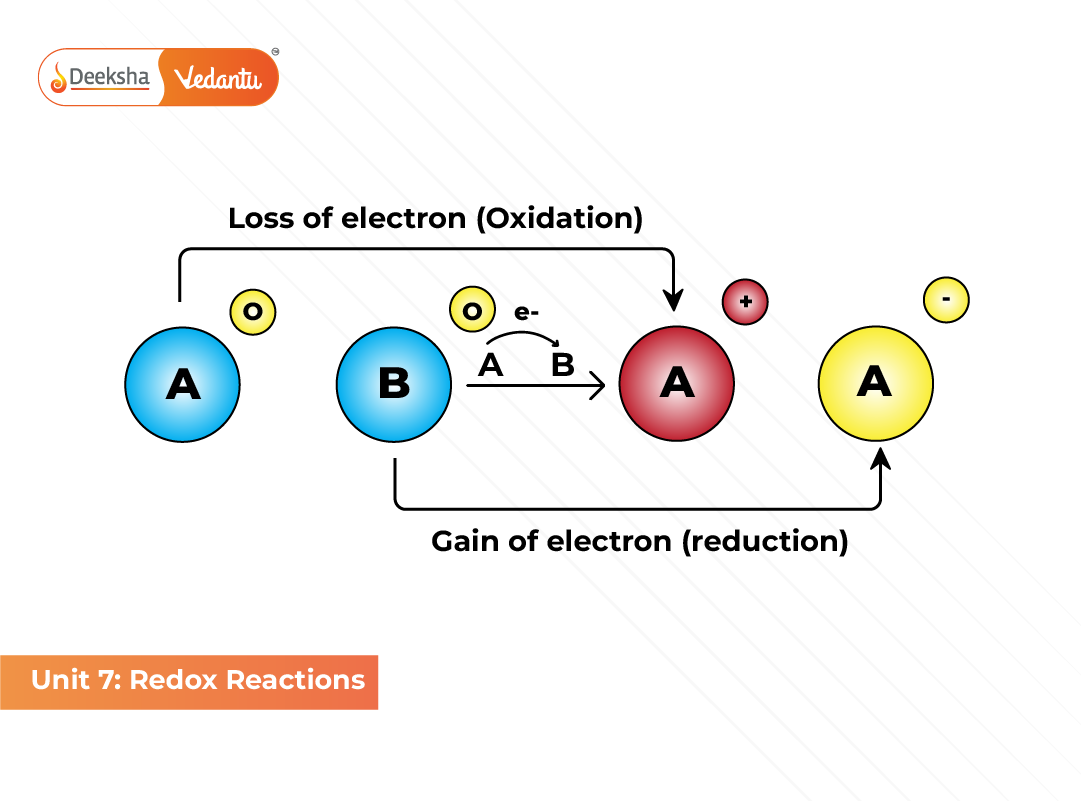
The term redox is derived from the combination of two processes:
- Oxidation: The process of electron loss by a substance.
- Reduction: The process of electron gain by a substance.
When oxidation and reduction occur simultaneously, the overall reaction is known as a redox reaction. The species that loses electrons is called the reducing agent, while the one that gains electrons is the oxidizing agent.
For example:
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
Here, zinc loses electrons (oxidation), and copper ions gain electrons (reduction). Thus, zinc acts as a reducing agent, and copper ions act as an oxidizing agent.
Oxidation and Reduction – Classical and Modern Concepts
In earlier chemistry, oxidation was defined as the addition of oxygen or removal of hydrogen, while reduction was defined as the removal of oxygen or addition of hydrogen.
Example:
CuO + H₂ → Cu + H₂O
- CuO loses oxygen → reduced to Cu (reduction)
- H₂ gains oxygen → oxidized to H₂O (oxidation)
However, modern chemistry redefined oxidation and reduction based on electron transfer:
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Hence, oxidation and reduction always occur together.
Oxidation Number and Oxidation State
To systematically identify which element is oxidized or reduced, the concept of oxidation number (O.N.) is used. The oxidation number represents the apparent charge an atom would have if electrons in a compound were assigned completely to the more electronegative atom.
Rules for Assigning Oxidation Numbers
- The O.N. of elements in their elemental state is 0.
(e.g., O₂, H₂, N₂, P₄ → O.N. = 0) - For monatomic ions, O.N. equals the ionic charge.
(e.g., Na⁺ = +1, Cl⁻ = -1) - Oxygen usually has O.N. = -2, except in peroxides (-1) and superoxides (-½).
- Hydrogen is +1 when bonded to nonmetals, and -1 when bonded to metals.
- The sum of oxidation numbers of all atoms in a neutral compound is 0; in a polyatomic ion, it equals the ion charge.
Example Calculations
- H₂SO₄: 2(+1) + S + 4(-2) = 0 → S = +6
- KMnO₄: (+1) + Mn + 4(-2) = 0 → Mn = +7
- Na₂O₂: 2(+1) + 2(-1) = 0 → O.N. of O = -1
Types of Redox Reactions
Redox reactions can be classified based on how oxidation and reduction occur:
1. Combination Reactions
Two or more reactants combine to form a single product.
Example:
2H₂ + O₂ → 2H₂O
2. Decomposition Reactions
A compound breaks down into simpler substances.
Example:
2KClO₃ → 2KCl + 3O₂
3. Displacement Reactions
An element in a compound is displaced by another element.
- Metal Displacement:
Fe + CuSO₄ → FeSO₄ + Cu - Non-Metal Displacement:
Cl₂ + 2NaBr → 2NaCl + Br₂
4. Disproportionation Reactions
A single substance undergoes both oxidation and reduction simultaneously.
Example:
2H₂O₂ → 2H₂O + O₂
Here, oxygen is both reduced (from -1 to -2) and oxidized (from -1 to 0).
Balancing Redox Reactions
Balancing redox reactions ensures that both mass and charge are conserved. There are two primary methods:
1. Oxidation Number Method
- Identify oxidation number changes.
- Balance the increase and decrease in oxidation numbers by multiplying with appropriate factors.
- Balance remaining atoms and charges.
Example:
Fe²⁺ + Cr₂O₇²⁻ + H⁺ → Fe³⁺ + Cr³⁺ + H₂O
Here, Fe²⁺ → Fe³⁺ (oxidation), and Cr₂O₇²⁻ → Cr³⁺ (reduction). The balanced equation becomes:
6Fe²⁺ + Cr₂O₇²⁻ + 14H⁺ → 6Fe³⁺ + 2Cr³⁺ + 7H₂O
2. Ion-Electron (Half-Reaction) Method
- Split the overall reaction into two half-reactions: oxidation and reduction.
- Balance each half for atoms and charge.
- Combine the two halves so that electrons cancel out.
This method is commonly used in electrochemistry and aqueous redox processes.
Redox Reactions and Electrode Processes
Electrochemical cells are devices that convert chemical energy into electrical energy (or vice versa) through redox reactions. These cells consist of two half-cells:
- Anode: Site of oxidation (electron loss)
- Cathode: Site of reduction (electron gain)
The electron flow from anode to cathode through an external circuit generates current. Understanding redox reactions forms the foundation of electrochemistry, including galvanic and electrolytic cells.
Applications of Redox Reactions
Redox reactions are essential across chemistry, biology, and industry:
- Metallurgy: Extraction of metals involves reduction of ores using carbon or electrolysis.
- Corrosion: Rusting of iron is a slow redox process involving oxygen and moisture.
- Energy Production: Combustion and respiration are redox processes that release energy.
- Photography and Batteries: Silver halide reduction and redox reactions in batteries are practical uses.
- Environmental Chemistry: Oxidation-reduction reactions are vital in wastewater treatment and pollution control.
FAQs
Q1. What are redox reactions in simple terms?
Redox reactions are chemical processes involving the simultaneous occurrence of oxidation (loss of electrons) and reduction (gain of electrons). These reactions are essential in energy transfer, respiration, and various industrial applications.
Q2. How can we identify a redox reaction?
A reaction can be identified as redox if there is a change in oxidation number of one or more elements between the reactants and products, indicating the transfer of electrons.
Q3. What is the difference between oxidizing and reducing agents?
An oxidizing agent gains electrons and is reduced in the process, whereas a reducing agent loses electrons and is oxidized.
Q4. What are the main types of redox reactions?
The main types are combination, decomposition, displacement, and disproportionation reactions. Each involves electron transfer or changes in oxidation state.
Q5. How are redox reactions balanced?
They can be balanced using two standard methods – the oxidation number method and the ion-electron (half-reaction) method. Both ensure conservation of mass and charge.
Q6. What are some real-life examples of redox reactions?
Examples include rusting of iron, photosynthesis, respiration, combustion of fuels, and reactions in batteries and electrochemical cells.
Q7. Why are redox reactions important in biology and industry?
In biology, redox reactions power essential processes like respiration and photosynthesis. In industry, they are crucial for metal extraction, corrosion prevention, energy generation, and environmental treatment.
Conclusion
Redox reactions are among the most fundamental processes in chemistry, governing energy flow, metabolism, and industrial chemistry. Understanding oxidation states, balancing techniques, and electron transfer mechanisms helps students comprehend complex chemical and electrochemical systems. Mastering this unit builds a strong foundation for future topics such as electrochemistry, thermodynamics, and coordination compounds-key areas for NEET and JEE aspirants.





Get Social