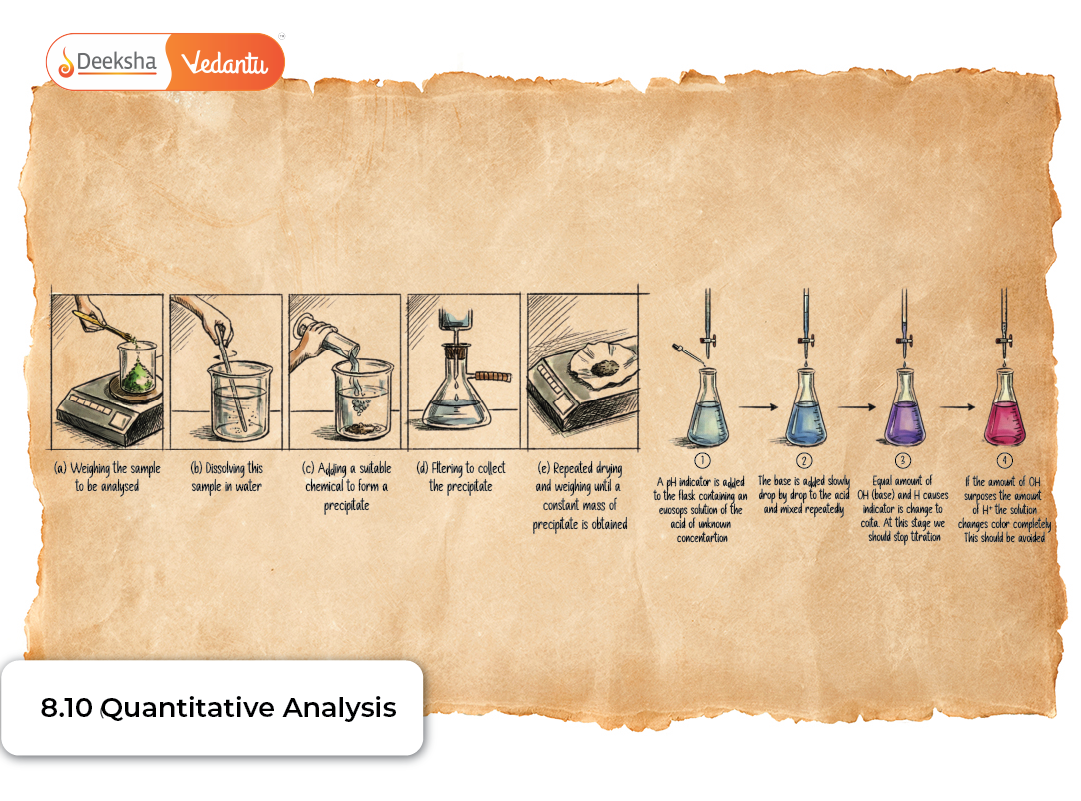
Quantitative analysis deals with determining the exact proportion of elements present in an organic compound. While qualitative analysis identifies which elements are present, quantitative analysis tells us how much of each element is present. This is essential for deriving empirical and molecular formulas and building a precise understanding of the compound’s composition.
At Deeksha Vedantu, students learn these analytical techniques with deep conceptual clarity, step‑by‑step guidance, and numerical practice-making even complex estimations feel intuitive.
Importance of Quantitative Analysis
Understanding the composition of an organic compound is crucial because:
- It forms the basis of formula determination.
- It helps classify unknown compounds.
- It supports purity checks.
- It is vital for pharmaceuticals, food chemistry, polymers, agriculture, and environmental studies.
NCERT Class 11 outlines quantitative estimation for:
- Carbon & Hydrogen
- Nitrogen
- Halogens (Cl, Br, I)
- Sulphur
- Phosphorus
Each element has a specific method based on how it reacts under oxidising or reducing conditions.
Estimation of Carbon and Hydrogen (Liebig’s Combustion Method)
This classical technique remains the foundation of organic elemental analysis.
Principle
The organic compound is burnt in excess oxygen. During this process:
- Carbon → CO₂
- Hydrogen → H₂O
These products are absorbed in:
- KOH for CO₂
- CaCl₂ for H₂O
The increase in mass of each absorbent tells us how much CO₂ and H₂O were formed.
Detailed Setup
A combustion furnace with:
- Pure dry oxygen supply
- Copper(II) oxide catalyst
- U‑tubes containing CaCl₂ (for H₂O)
- U‑tubes containing KOH (for CO₂)
Reaction Summary
C + O₂ → CO₂
2H + ½ O₂ → H₂O
Steps
- Weigh the sample.
- Burn completely with CuO in a combustion furnace.
- Collect CO₂ and H₂O in absorption tubes.
- Reweigh the tubes to find the gain in mass.
Calculations
- %C = (12 × mass of CO₂) / (44 × mass of sample) × 100
- %H = (2 × mass of H₂O) / (18 × mass of sample) × 100
Elaborated NCERT‑Style Example
- 0.4 g of compound gives 0.88 g CO₂ and 0.36 g H₂O.
- Carbon: % C = (12/44 × 0.88 / 0.4) × 100 = 60%
- Hydrogen: % H = (2/18 × 0.36 / 0.4) × 100 = 10%
- Thus, 70% of the compound is C and H; the remaining might be O, N, or halogens.
Exam Tip
If the sum of %C and %H is unusually low, the compound likely contains halogens. If high, the compound may be oxygen-rich.
Estimation of Nitrogen
Nitrogen is estimated using two classical methods: Dumas and Kjeldahl.
A. Dumas Method
This method is fast and suitable for automation.
Principle
The sample is heated strongly with copper oxide at high temperature, converting nitrogen to elemental nitrogen (N₂ gas).
The N₂ is collected, purified from CO₂ and moisture, and measured.
Key Steps
- Burn compound with excess CuO.
- Remove CO₂ by passing gas through KOH.
- Dry the gas.
- Collect N₂ in a graduated tube.
Calculation
% N = (Volume of N₂ × 28) / (22.4 × mass of sample) × 100
When preferred
- Best for heat-stable compounds
- Very accurate for proteins, pharmaceuticals
B. Kjeldahl’s Method
A widely used method for nitrogen determination-especially in food chemistry.
Principle
- Nitrogen is converted into ammonium sulphate by heating with concentrated H₂SO₄.
- (N in compound) + H₂SO₄ → (NH₄)₂SO₄
- Then (NH₄)₂SO₄ + 2 NaOH → 2 NH₃ + Na₂SO₄ + 2 H₂O
- The ammonia evolved is absorbed in known excess of acid.
Calculation
% N = (1.4 × V × N) / mass of sample Where:
- 1.4 is a constant derived from molar relations
- V = volume of acid consumed
- N = normality
Limitations
Does not detect nitrogen in:
- Nitro compounds (–NO₂)
- Azo compounds (–N=N–)
- Nitrogen in aromatic rings These resist conversion to ammonium salts.
Exam Insight
In most competitive exams, students must choose which method is appropriate based on the functional group.
Estimation of Halogens (Carius Method)
Halogens (Cl, Br, I) are determined gravimetrically using the Carius method.
Principle
The compound is oxidised using fuming nitric acid and silver nitrate inside a sealed Carius tube at high temperature.
Halogens form insoluble silver halides (AgX):
- AgCl → white
- AgBr → pale yellow
- AgI → yellow
Steps
- Place organic compounds in a Carius tube.
- Add fuming HNO₃ and AgNO₃.
- Heat in a furnace.
- Cool and filter AgX.
- Wash, dry, and weigh.
Calculation
% Halogen = (Atomic mass of halogen × mass of AgX) / (Atomic mass of Ag × mass of sample) × 100
Example
- A 0.3 g compound yields 0.95 g AgBr.
- % Br = (80 × 0.95) / (108 × 0.3) × 100 ≈ 234 / 32.4 ≈ 72.2%
- This high % suggests the compound is bromine‑rich (likely an alkyl bromide).
Estimation of Sulphur
Sulphur is also estimated using Carius oxidation.
Principle
- Sulphur → Sulphuric acid (H₂SO₄)
- H₂SO₄ + BaCl₂ → BaSO₄ (white ppt)
- The BaSO₄ formed is filtered, washed, dried, and weighed.
Calculation
% S = (32 × mass of BaSO₄) / (233 × mass of sample) × 100
Example
- 0.2 g compound produces 0.4 g BaSO₄.
- % S = (32 × 0.4) / (233 × 0.2) × 100 = (12.8 / 46.6) × 100 ≈ 27.5%
- A value this high suggests a thiol or thioether.
Exam Tip
If BaSO₄ formed is low, sulphur may be part of a sulphone or sulphonamide, which oxidises less efficiently.
Estimation of Phosphorus
Phosphorus is important in biomolecules like DNA, ATP, and pesticides.
Principle
- Phosphorus is oxidised to phosphoric acid (H₃PO₄) using fuming nitric acid.
- It forms yellow ammonium phosphomolybdate when reacted with ammonium molybdate.
Steps
- Oxidise compound with fuming HNO₃.
- Convert phosphorus into H₃PO₄.
- Add ammonium molybdate.
- Filter yellow precipitate.
- Dry and weigh.
Calculation
% P = (31 × mass of precipitate) / (M × mass of sample) × 100 Where M = molar mass of the phosphomolybdate compound.
Example
If 0.3 g of sample gives 0.9 g phosphomolybdate: % P = (31 × 0.9) / (1435 × 0.3) × 100 ≈ 6.5%
Summary Table
| Element | Method | Key Principle |
| Carbon & Hydrogen | Liebig combustion | Oxidation to CO₂ and H₂O |
| Nitrogen | Dumas | Conversion to N₂ gas |
| Nitrogen | Kjeldahl | Conversion to NH₃ absorbed in acid |
| Halogens | Carius | Precipitation as AgX |
| Sulphur | Carius | Oxidation to BaSO₄ |
| Phosphorus | Phosphomolybdate | Formation of yellow ppt |
Deep‑Dive Explanations and Examples
Why Different Elements Need Different Estimation Methods
Each element in an organic compound behaves differently when oxidised, heated, or treated with acids and bases. For example:
- Carbon and hydrogen burn cleanly to CO₂ and H₂O.
- Nitrogen may form ammonia, nitrogen gas, or nitrogen oxides depending on conditions.
- Halogens form insoluble silver halides only after strong oxidation.
- Sulphur must be forced into the sulphate form for accurate measurement.
- Phosphorus requires conversion to phosphomolybdate because it does not form easily isolable simple salts.
Understanding these distinctions helps students answer reasoning questions in competitive exams.
Why Combustion Must Be Complete in Liebig’s Method
If combustion is incomplete:
- Carbon might form CO instead of CO₂.
- Hydrogen might remain unoxidised.
This leads to underestimation of carbon and hydrogen. Hence, a copper(II) oxide catalyst and continuous O₂ flow ensure complete combustion.
Why Dumas Method Removes CO₂ Before Measuring N₂
If CO₂ is left in the gas mixture, the measured gas volume would increase artificially. Since CO₂ is absorbed by KOH, passing the gas through KOH ensures that only nitrogen gas is collected.
Why Kjeldahl’s Method Fails for Nitro and Azo Compounds
These compounds contain nitrogen in oxidation states and bonding arrangements that resist conversion to ammonium sulphate. They do not break down under typical Kjeldahl digestion conditions.
Why Carius Tube Is Essential for Halogen/Sulphur Analysis
Fuming nitric acid and silver nitrate react violently at high temperatures. Ordinary glassware would:
- Melt
- Crack
- Fail to withstand pressure
Carius tubes are made of thick‑walled hard glass that can tolerate extreme conditions.
Solved Numericals
Example 1: Calculate %C and %H
- A 0.3 g organic compound produced 0.66 g CO₂ and 0.27 g H₂O.
- Carbon: % C = (12/44 × 0.66 / 0.3) × 100 ≈ 60%
- Hydrogen: % H = (2/18 × 0.27 / 0.3) × 100 ≈ 10%
Example 2: Dumas Nitrogen Estimation
- 10 mL N₂ was collected at STP from 0.15 g sample.
- % N = (10 × 28) / (22.4 × 0.15) × 100 ≈ 83.3%
Example 3: Kjeldahl Nitrogen Estimation
- A 0.4 g sample required 20 mL of 0.1 N HCl to neutralise released NH₃.
- % N = (1.4 × 20 × 0.1) / 0.4 = 7%
Example 4: Halogen Estimation (Carius)
- 0.25 g compound gives 0.7 g AgCl.
- % Cl = (35.5 × 0.7) / (108 × 0.25) × 100 ≈ 92.0%
Example 5: Sulphur Estimation
- 0.18 g sample yields 0.36 g BaSO₄.
- % S = (32 × 0.36) / (233 × 0.18) × 100 ≈ 27.5%
Exam‑Focused Insights
Common Traps in JEE/NEET Questions
- Incorrect molar ratios in combustion analysis.
- Ignoring absorbed water in CaCl₂ tubes.
- Wrong gas volume corrections in Dumas questions.
- Forgetting Kjeldahl limitations.
- Confusing AgCl, AgBr, AgI masses.
Quick Memory Aids
- KOH absorbs CO₂, CaCl₂ absorbs H₂O.
- Dumas gives N₂, Kjeldahl gives NH₃.
- Halogens → AgX; heavier the halogen, deeper the colour.
- Sulphur → BaSO₄, Phosphorus → yellow phosphomolybdate.
FAQs
Q1. Why are different estimation methods used for different elements?
Because each element behaves differently during oxidation or thermal decomposition. Some form gases (N₂), some form precipitates (AgX, BaSO₄), and some require special reagents.
Q2. How can oxygen percentage be determined in an organic compound?
Oxygen is not estimated directly. Its percentage is calculated by subtracting the total percentages of other elements from 100.
Q3. Why is Kjeldahl’s method unsuitable for nitro and azo compounds?
Nitrogen in these groups is not converted into ammonium sulphate during digestion, so it cannot be detected using Kjeldahl’s method.
Q4. Why is a Carius tube used for halogen and sulphur estimation?
Because reactions involve high temperatures and corrosive reagents; the Carius tube withstands pressure and prevents explosion or leakage.
Q5. What is the most common source of error in combustion analysis?
Incomplete combustion, which leads to lower values of CO₂ and H₂O, causing underestimation of carbon and hydrogen.
Conclusion
Quantitative analysis offers students a complete understanding of the composition of organic compounds. With methods like Liebig’s combustion, Dumas nitrogen estimation, Kjeldahl digestion, and Carius halogen/sulphur analysis, students gain accurate numerical skills needed for empirical and molecular formula calculations. Deeksha Vedantu’s structured explanations, deep reasoning, and practice numericals equip learners with the clarity and confidence required to excel in exams.










Get Social