Matter is everything that surrounds us — the air we breathe, the water we drink, the chair we sit on, and even our own bodies. It has both mass and volume, and understanding its nature is the cornerstone of chemistry. From atomic interactions to chemical bonding, every principle in chemistry is built upon the study of matter. For JEE aspirants, mastering this chapter is crucial since it provides the theoretical framework for upcoming topics like thermodynamics, atomic structure, and chemical bonding.
The nature of matter not only describes how matter exists but also how it changes under varying conditions of temperature and pressure. The study extends beyond visible materials to include microscopic particles and quantum states, forming the foundation for both Physical Chemistry and Modern Physics.
States of Matter
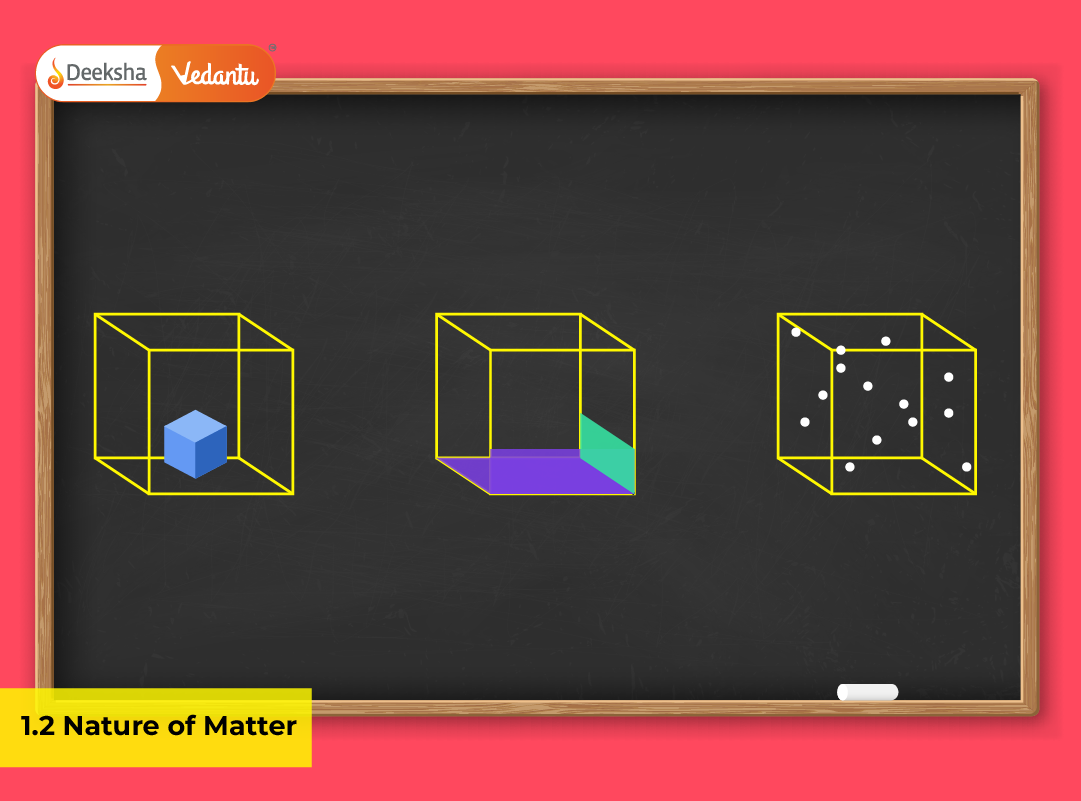
Matter exists in several physical forms called states, determined by the arrangement and motion of particles. The main three states are solid, liquid, and gas, while plasma and Bose-Einstein condensate (BEC) are recognized in advanced physical contexts.
Solid State
- Definite shape and volume: Solids retain their dimensions because particles are closely packed in fixed positions.
- Strong intermolecular forces: The force of attraction keeps the particles in place, allowing only vibrational motion.
- Incompressibility: Minimal empty space means solids cannot be compressed easily.
- Examples: Metals, salt, ice, glass.
JEE Insight: Concepts like crystal lattice, unit cells, and types of solids (ionic, molecular, covalent, metallic) are vital for questions in Physical Chemistry. Understanding density and packing efficiency is essential.
Sample Problem:
A cube of copper has a side of 4 cm and weighs 356 g. Find its density.
Solution: Density = Mass / Volume = 356 / (4³) = 356 / 64 = 5.56 g/cm³.
Additional Concept: Some solids like glass and plastic are amorphous (no fixed structure), while crystals such as salt are crystalline solids with orderly arrangements.
Liquid State
- Fixed volume but variable shape: Liquids assume the container’s shape but maintain constant volume.
- Moderate intermolecular forces: Particles slide past each other, leading to fluidity.
- Incompressibility and flow: Liquids exhibit viscosity and surface tension.
- Examples: Water, mercury, oil.
Applications in JEE: Questions can cover vapor pressure, viscosity, and surface tension, especially in thermodynamic systems involving liquids. Students should link these with kinetic energy and intermolecular attraction.
Sample Problem:
Explain why water forms spherical droplets on a leaf surface.
Answer: Due to surface tension, molecules on the surface experience inward forces that minimize surface area, leading to spherical droplets.
Real-World Relevance: Understanding liquids helps explain phenomena like capillary rise, detergents, and oil-water separation.
Gaseous State
- No definite shape or volume: Gases fill any available space.
- Weak intermolecular forces: Particles move freely and rapidly.
- High compressibility: Volume changes significantly with pressure.
- Examples: Oxygen, nitrogen, carbon dioxide.
Equation of State: PV = nRT (Ideal Gas Law) — linking pressure, volume, moles, and temperature.
JEE Focus: The gaseous state is one of the most tested topics. Students should master Boyle’s, Charles’s, and Avogadro’s laws, as well as real gas behavior using the Van der Waals equation.
Sample Problem:
If 1 mole of an ideal gas occupies 22.4 L at STP, find its pressure at 300 K when volume = 24.6 L.
Solution: Using PV/T = constant → P = (22.4 × 273) / (24.6 × 300) = 0.83 atm.
Additional Note: Gaseous laws form a bridge between macroscopic thermodynamics and molecular kinetics, crucial for JEE’s conceptual questions.
Other States of Matter
- Plasma: Consists of ions and free electrons; found in lightning, stars, and fluorescent lights.
- Bose-Einstein Condensate (BEC): A state formed at near-zero temperatures when atoms condense into a single quantum state.
JEE Application: Advanced problems may involve plasma in astrophysics or BEC in thermodynamics extensions.
Classification of Matter
Matter can be classified based on physical state, composition, and chemical nature. This helps in understanding chemical reactivity, mixture behavior, and molecular interactions.
Physical Classification
The simplest classification divides matter into solids, liquids, and gases, based on physical properties like volume, shape, and particle mobility.
Chemical Classification
Chemically, matter is categorized as pure substances and mixtures.
Pure Substances
A pure substance has a definite composition and unique chemical behavior.
- Elements: Made up of identical atoms. Examples: Iron (Fe), Hydrogen (H₂), Gold (Au).
- Compounds: Contain two or more elements in fixed ratios. Examples: Water (H₂O), Carbon dioxide (CO₂).
JEE Application: Identifying compounds using percentage composition and molecular formula is a frequent exercise.
Sample Problem:
Water contains hydrogen and oxygen in an 1:8 ratio by mass. If you have 36 g of water, how much oxygen is present?
Solution: (Oxygen/Total) = 8/9 → Oxygen = 36 × 8/9 = 32 g.
Mixtures
Mixtures contain two or more substances physically combined.
- Homogeneous Mixtures: Same composition throughout (e.g., air, sugar solution).
- Heterogeneous Mixtures: Non-uniform composition (e.g., oil and water, granite).
Separation Techniques:
- Filtration → separates solids from liquids.
- Distillation → separates liquids by boiling points.
- Chromatography → separates components based on adsorption.
JEE Tip: Be familiar with fractional distillation and solvent extraction as they form conceptual bases for chemical industries.
JEE Weightage and Applications
| Topic | Average Weightage | Common JEE Question Areas | Concept Linkages |
| States of Matter | 2–3 marks | Gas laws, kinetic theory, deviation from ideal behavior | Thermodynamics, Chemical Kinetics |
| Classification of Matter | 1–2 marks | Pure vs Mixture analysis, separation processes | Atomic Structure, Stoichiometry |
Understanding this unit thoroughly allows students to connect basic physical concepts with thermodynamics, mole concept, and equilibrium, which together carry 10–15 marks in JEE Main.
Summary
The study of matter covers both its physical forms and chemical classification. From rigid solids to dynamic gases, matter exhibits behaviors driven by intermolecular forces and energy interactions. Recognizing whether a substance is pure or a mixture helps predict its behavior in reactions and physical changes. These principles serve as a foundation for nearly all physical and chemical laws encountered in higher chapters.
FAQs
Q1. What matters in chemistry?
Matter is anything that occupies space and possesses mass — it can exist as solid, liquid, gas, or plasma.
Q2. What factors decide the state of matter?
Temperature, pressure, and the strength of intermolecular forces together determine whether matter exists as a solid, liquid, or gas.
Q3. What is the main difference between an element and a compound?
An element consists of one type of atom, while a compound has atoms of two or more elements chemically bonded in fixed proportions.
Q4. How are homogeneous and heterogeneous mixtures different?
Homogeneous mixtures are uniform throughout, while heterogeneous mixtures have visibly distinct components.
Q5. Which subtopic of ‘Nature of Matter’ is most important for JEE?
Gas laws, ideal gas equation, and classification of matter questions often appear in the basic concepts of Physical Chemistry, forming the base for stoichiometry and equilibrium.
Q6. Why are intermolecular forces significant in JEE questions?
They explain phenomena such as phase transitions, boiling points, viscosity, and solubility, all of which are common JEE concepts.
Q7. How does this topic link to real-life applications?
Understanding states and classification helps explain natural and industrial processes such as water purification, air separation, and alloy formation.
Conclusion
The Nature of Matter provides the backbone for understanding all chemical and physical processes. For JEE aspirants, this chapter strengthens conceptual clarity that connects to nearly every topic in chemistry — from thermodynamics to chemical bonding. By visualizing how matter behaves under various conditions, students can easily solve numerical problems, interpret molecular structures, and relate microscopic phenomena to macroscopic observations. This foundation ensures success not only in JEE exams but also in advanced scientific reasoning and problem-solving in higher studies.





Get Social