The mole concept is one of the most fundamental and powerful ideas in chemistry. It connects the microscopic world of atoms and molecules with the macroscopic quantities we measure in the laboratory. The mole serves as a bridge, allowing chemists to count atoms, molecules, or ions in a given mass of substance. Understanding this concept thoroughly is essential for solving stoichiometric problems, determining empirical and molecular formulas, and balancing chemical equations—all crucial for JEE and NEET aspirants.
Definition of a Mole
A mole is defined as the amount of substance that contains as many elementary entities (atoms, molecules, ions, or electrons) as there are atoms in 12 grams of pure carbon-12 (¹²C). This number is known as Avogadro’s number (Nₐ).
Avogadro’s number (Nₐ) = 6.022 × 10²³ particles/mol
This means that:
- 1 mole of oxygen atoms = 6.022 × 10²³ atoms of oxygen
- 1 mole of water molecules = 6.022 × 10²³ molecules of water
- 1 mole of sodium chloride = 6.022 × 10²³ formula units of NaCl
The mole concept provides a universal counting unit for chemists, similar to how we use “dozen” to count 12 items.
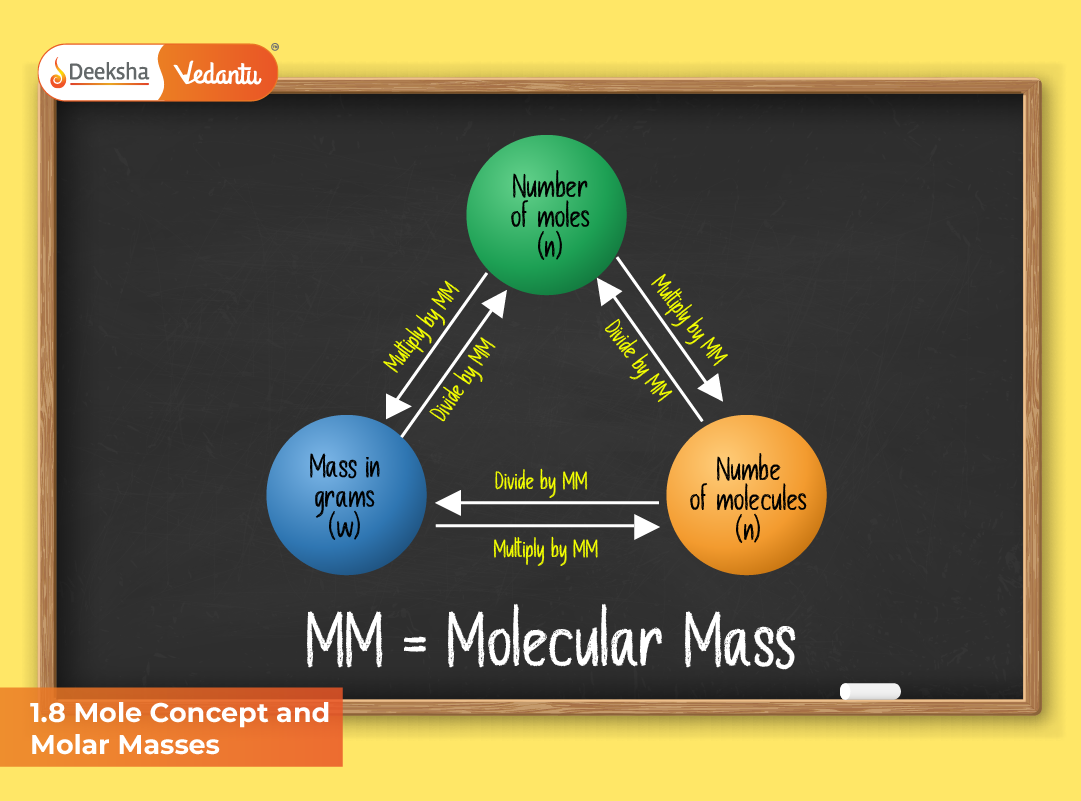
Relationship Between Mole, Mass, and Number of Particles
The following relationships summarize the mole concept:
| Quantity | Symbol | Relationship |
| Number of moles | n | n = Given mass (g) / Molar mass (g/mol) |
| Number of particles | N | N = n × Nₐ |
| Given mass | m | m = n × Molar mass |
| Molar mass | M | M = m / n |
Example:
Find the number of moles in 18 g of water (H₂O).
Molar mass of H₂O = 18 g/mol
Number of moles (n) = m / M = 18 / 18 = 1 mol
Hence, 18 g of water contains 1 mole of H₂O molecules, or 6.022 × 10²³ molecules.
Molar Mass
Molar mass is defined as the mass of one mole of a substance. It is numerically equal to the atomic or molecular mass but expressed in grams per mole (g/mol).
Formula:
Molar mass = Mass of one mole of substance
Examples:
- Molar mass of hydrogen (H₂) = 2 g/mol
- Molar mass of oxygen (O₂) = 32 g/mol
- Molar mass of sodium chloride (NaCl) = 58.5 g/mol
Thus, 1 mole of NaCl weighs 58.5 g and contains 6.022 × 10²³ formula units.
JEE Tip: Always verify whether the given mass refers to atomic, molecular, or formula mass before applying the molar mass formula.
Interconversion of Moles, Mass, and Number of Particles
You can easily move between different measurable quantities using the mole as a central link.
| To Find | Formula |
| Mass from moles | m = n × M |
| Moles from mass | n = m / M |
| Number of particles from moles | N = n × Nₐ |
| Moles from number of particles | n = N / Nₐ |
Example 1:
How many atoms are present in 4 g of hydrogen gas (H₂)?
n = m / M = 4 / 2 = 2 mol
Number of molecules = 2 × 6.022 × 10²³ = 1.204 × 10²⁴ molecules
Since each molecule has 2 atoms, total atoms = 2 × 1.204 × 10²⁴ = 2.408 × 10²⁴ atoms.
Example 2:
How many moles are there in 22 g of CO₂?
Molar mass of CO₂ = 44 g/mol
n = 22 / 44 = 0.5 mol
Thus, the sample contains 0.5 mol or 3.011 × 10²³ molecules.
Concept of Gram Atomic, Gram Molecular, and Gram Formula Mass
- Gram Atomic Mass: Mass of one mole of atoms of an element in grams. (e.g., 1 mole of O atoms = 16 g)
- Gram Molecular Mass: Mass of one mole of molecules in grams. (e.g., 1 mole of H₂O = 18 g)
- Gram Formula Mass: Mass of one mole of ionic formula units. (e.g., 1 mole of NaCl = 58.5 g)
These terms are used interchangeably with molar mass depending on the context.
Importance of the Mole Concept
- Simplifies chemical calculations: Enables conversion between microscopic particles and macroscopic quantities.
- Used in stoichiometry: Helps in balancing equations and calculating reactant and product masses.
- Determines empirical and molecular formulas: Based on percentage composition data.
- Connects laboratory data with theory: Allows real-world measurement of atomic-level phenomena.
JEE Insight: Many numerical problems in physical and inorganic chemistry rely on accurate application of the mole concept. Master formulas and unit conversions thoroughly.
FAQs
Q1. What is the mole concept in simple terms?
The mole concept relates the number of particles in a substance to its measurable mass. One mole contains 6.022 × 10²³ particles of that substance.
Q2. Why is Avogadro’s number important?
Avogadro’s number acts as a bridge between atoms and grams, allowing chemists to count particles in macroscopic quantities.
Q3. What is the unit of molar mass?
Molar mass is expressed in grams per mole (g/mol).
Q4. How can we calculate the number of particles from mass?
Use the formula: Number of particles = (Given mass / Molar mass) × Avogadro’s number.
Q5. How does the mole concept help in stoichiometry?
It allows you to determine how many moles of reactants combine to produce a specific number of moles of products in a balanced equation.
Q6. What is the difference between atomic mass and molar mass?
Atomic mass is the mass of a single atom (in amu), while molar mass is the mass of one mole of atoms (in g/mol).
Conclusion
The mole concept and molar masses form the quantitative core of chemistry. By mastering the relationships between moles, mass, and number of particles, students can easily solve complex chemical calculations. For JEE and NEET, understanding these relationships ensures strong command over stoichiometry, gas laws, and chemical equations — all of which are heavily tested in exams.





Get Social