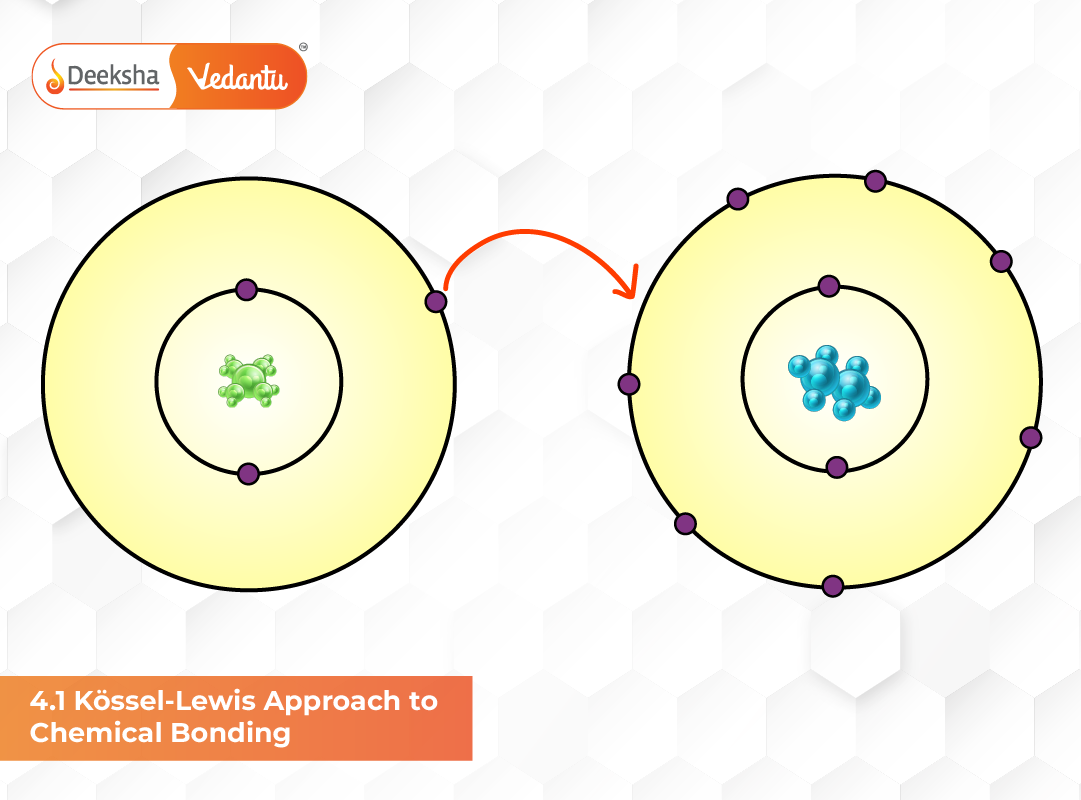
Chemical bonding plays a fundamental role in determining how elements exist in nature and interact with each other. The Kössel–Lewis approach marked a major turning point in chemical science by providing the first modern and systematic explanation for bond formation. According to this theory, atoms achieve stability by either gaining, losing, or sharing electrons. This idea is based on their tendency to attain the electronic configuration of noble gases, which are chemically inert due to their stable valence shells. This concept forms the foundation of Unit 4 of Class 11 NCERT Chemistry, making it essential for students preparing for board exams, NEET, and JEE.
The Kössel–Lewis approach simplified understanding chemical bonding by visually representing valence electrons, predicting bond types, and explaining molecular stability. It paved the way for advanced chemical theories like VSEPR Theory, Valence Bond Theory, Molecular Orbital Theory, and Hybridisation. Mastering this chapter builds logical understanding and problem-solving skills for chemical structures and bond-related numerical questions.
Importance of Kössel–Lewis Approach
The Kössel–Lewis approach helps in explaining why atoms combine and how molecules are formed. It provides clarity regarding the role of valence electrons and explains why reactions occur. The approach is useful for understanding:
- Why elements react to achieve stability
- Formation of ionic and covalent bonds
- The tendency of atoms to achieve noble gas configuration
- Role of valence electrons in bond formation
- Visual representation of atoms using Lewis symbols
- Foundation of the octet rule
This theory creates a bridge between atomic structure and molecular formation, making chemical bonding easier to understand for beginners. It is used in almost every topic of physical, inorganic, and organic chemistry, and hence is widely applicable in higher-level studies.
Kössel’s Explanation – Formation of Ionic Bonds
Kössel focused on elements located near the extremes of the periodic table. These elements either lose or gain electrons to attain stability. Metals, being electropositive, easily lose electrons and form cations, whereas non-metals gain electrons to form anions. The strong electrostatic force of attraction between these oppositely charged ions leads to the formation of ionic bonds.
Key Points of Kössel’s Theory:
- Noble gases such as He, Ne, and Ar possess stable configurations and show minimal chemical activity.
- Metals like Na, K, Mg, Ca lose electrons and form positively charged ions.
- Non-metals like O, F, Cl, Br gain electrons to form negatively charged ions.
- Ionic bond formation is driven by attaining noble gas configuration.
- The resulting ionic compound forms a crystalline lattice structure.
Example: Formation of Sodium Chloride (NaCl)
Electron transfer explanation:
Na (2,8,1) → Na⁺ (2,8) + e⁻
Cl (2,8,7) + e⁻ → Cl⁻ (2,8,8)
Na⁺ attracts Cl⁻ → NaCl solid
This bonding produces a rigid ionic lattice that exhibits high melting point, solubility in water, and good electrical conductivity in molten or aqueous states.
Lewis’ Explanation – Formation of Covalent Bonds
Lewis expanded the theory by explaining that atoms can also share valence electrons instead of gaining or losing them. This type of bonding is known as covalent bonding. Lewis introduced electron-dot structures, now called Lewis structures, to represent valence electrons visually.
Lewis Symbols (Electron-Dot Symbols)
Only valence electrons are displayed as dots around the element’s symbol:
- H: •H
- Cl: ••Cl•••
- C: •C•• (four valence electrons)
Lewis Structures (Electron-Dot Structures)
Atoms form bonds by sharing electrons to attain a stable noble gas configuration. This is represented using dots or dashes.
Example: Formation of H₂ molecule:
H• + •H → H–H
Each hydrogen atom shares one electron and attains a stable two-electron configuration similar to helium.
The Octet Rule – Fundamental of Chemical Bonding
The octet rule states:
Atoms tend to gain, lose, or share electrons until they have eight electrons in their valence shell.
This rule highlights the tendency of atoms to become stable like noble gases.
Examples Supporting the Octet Rule
| Molecule | Explanation | Bonding Type |
| CH₄ | Carbon shares 4 electron pairs with 4 hydrogens | Covalent |
| H₂O | Oxygen shares electrons with 2 hydrogens | Covalent |
| CO₂ | Carbon forms double bonds with oxygen | Covalent |
| NaCl | Electron transfer between Na and Cl | Ionic |
This rule successfully explains bonding in many molecules but also has limitations.
Steps to Draw Lewis Structures
The Lewis method is used to visually represent bonding in molecules. Follow these steps:
- Count total valence electrons.
- Write the basic skeletal structure.
- Place single bonds between atoms.
- Complete octet of surrounding atoms.
- Add leftover electrons as lone pairs.
- If required, convert lone pairs into double or triple bonds.
Examples of Lewis Structures
| Molecule | Representation | Explanation |
| H₂ | H–H | Both H atoms complete duet configuration |
| O₂ | O=O | Double bond ensures octet completion |
| N₂ | N≡N | Triple bond gives maximum stability |
| CO₂ | O=C=O | Linear structure with double bonds |
What Is Formal Charge?
When a molecule has multiple possible structures, formal charge helps determine the most stable one.
Formula:
Formal Charge = Valence e⁻ – Non-bonding e⁻ – ½ × Bonding e⁻
Example: Ozone (O₃)
One oxygen atom carries +1 charge, another –1 charge, while the third remains neutral. The total charge is zero, matching the real molecule. This helps in selecting the correct structure.
Limitations of the Octet Rule
The octet rule does not apply to all molecules. Several exceptions exist:
Cases Where Octet Rule Fails:
- Incomplete Octet – BF₃, BeCl₂
- Odd-Electron Molecules – NO, NO₂
- Expanded Octet – PF₅, SF₆, XeF₄
- No Explanation of Shape or Bond Energy
These exceptions show that while the octet rule is useful, it is not universally applicable, especially for transition metals and complex molecules.
Importance for Class 11 & Competitive Exams
The Kössel–Lewis approach forms the basis for understanding bonding theories used in further chapters. It prepares students for advanced topics:
- VSEPR Theory
- Valence Bond Theory
- Hybridisation
- Molecular Orbital Theory
- Resonance
This theory is also crucial for reaction mechanisms in organic chemistry and bond-energy calculations in physical chemistry. It holds strong relevance in NEET, JEE, and Class 11 examinations.
FAQs
Q1: How is Kössel’s theory different from Lewis’ theory?
Kössel explained bonding using electron transfer (ionic bonds), whereas Lewis explained bonding using electron sharing (covalent bonds).
Q2: What is the octet rule?
The octet rule states that atoms achieve stability when they possess eight electrons in their outermost shell.
Q3: Why is formal charge important in chemistry?
Formal charge helps determine the most stable Lewis structure when several structures are possible for a molecule.
Q4: Are all molecules able to follow the octet rule?
No, several molecules like BF₃ and NO do not follow the octet rule due to electronic limitations and exceptions.
Conclusion
The Kössel–Lewis approach provided the earliest and most structured explanation of chemical bonding, linking atomic structure to molecular formation. It showed how atoms work toward achieving stable configurations via electron transfer or sharing, leading to ionic and covalent bond formation. Although the octet rule has limitations, it remains a crucial concept for understanding bonding trends and predicting molecular behavior. This theory marks the foundation for all subsequent bonding models and plays an essential role in preparing students for advanced chemical concepts in Class 11 NCERT Chemistry and competitive exams.





Get Social