Chemical bonding lies at the heart of understanding chemical reactions, and the ionic or electrovalent bond is one of the most fundamental types of bonds explained in Class 11 NCERT Chemistry. It occurs when atoms transfer electrons to achieve stable noble gas configurations. This transfer leads to the formation of positively charged ions (cations) and negatively charged ions (anions), which are held together by strong electrostatic forces of attraction. Such bonding plays a crucial role in the formation of salts and minerals found in nature.
The study of ionic bonds helps in understanding the physical properties of compounds such as melting point, solubility, hardness, and conductivity. These concepts are highly relevant in chemical industries, biochemistry, metallurgy, and everyday life applications. Therefore, understanding this topic thoroughly is essential not only for board exams but also for entrance exams like NEET and JEE.
Definition of Ionic or Electrovalent Bond
Ionic bonding is one of the oldest and most widely studied bonding theories in chemistry. It plays a foundational role in explaining how atoms interact to form stable compounds. Rather than sharing electrons like in covalent bonds, ionic bonds involve a complete transfer of electrons from one atom to another. This process allows atoms to achieve a noble gas configuration, which is associated with maximum stability. Understanding ionic bonding gives insight into why certain reactions are highly exothermic and why some compounds exist as solids at room temperature while others are gases or liquids.
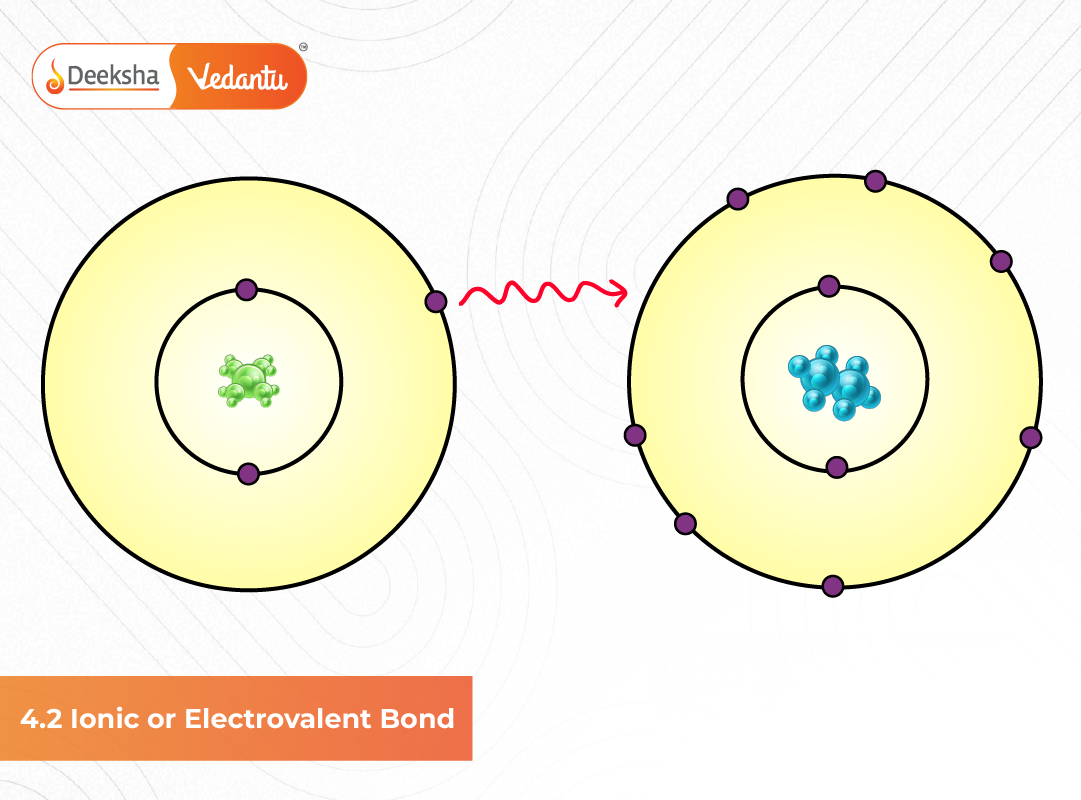
An ionic bond is formed when one atom transfers one or more electrons to another atom. The atom losing electrons becomes a cation, while the atom gaining electrons becomes an anion. The attraction between the opposite charges results in the formation of a stable ionic compound.
Why Do Ionic Bonds Form? (NCERT Logic)
Atoms always tend to become stable. Elements that have:
- 1, 2, or 3 electrons in their valence shell → prefer to lose electrons
- 5, 6, or 7 electrons in their valence shell → prefer to gain electrons
By losing or gaining electrons, atoms become ions, and these oppositely charged ions attract each other strongly, forming an ionic (electrovalent) bond.
Example:
Sodium (Na) has 1 electron in its outer shell → it loses this electron.
Chlorine (Cl) has 7 electrons in its outer shell → it gains 1 electron.
Together, they form NaCl (common salt) through an electrovalent bond.
Conditions for Ionic Bond Formation
The formation of an ionic bond does not happen randomly; it follows specific energetic and structural requirements. These conditions ensure that the transfer of electrons is energetically favourable and leads to the formation of a stable compound. In many biological, environmental, and industrial processes, the tendency of atoms to gain or lose electrons determines the reactivity of substances. Therefore, understanding these conditions is vital for predicting the feasibility of reactions and designing chemical processes in laboratories and industries.
For an ionic bond to be formed, certain criteria must be fulfilled:
- Large difference in electronegativity between two atoms.
- One atom must have low ionisation enthalpy (usually metals).
- The other should have high electron gain enthalpy (usually non-metals).
- Formation of a stable crystalline lattice.
Example: Formation of Sodium Chloride (NaCl)
The process of ionic bond formation in sodium chloride illustrates the driving force behind electron transfer. Sodium, being a highly reactive metal, readily loses an electron to achieve stability, while chlorine, a reactive non-metal, eagerly accepts electrons to complete its octet. This simple reaction is of great importance because it represents the basic principle of ionic bonding found in several biological salts, preservatives, and industrial chemicals. Additionally, NaCl is used as a reference compound when studying lattice energy and bond strength in chemistry.
Electron Transfer:
Na (2,8,1) → Na⁺ + e⁻
Cl (2,8,7) + e⁻ → Cl⁻
Na⁺ attracts Cl⁻ → NaCl (solid crystalline structure)
The strong attraction leads to a rigid lattice, giving ionic compounds their characteristic properties.
Formation of Ions
| Atom | Atomic No. | Electronic Configuration | Tendency | Ion Formed |
| Na (Sodium) | 11 | 2, 8, 1 | Loses 1 e⁻ | Na⁺ |
| Mg (Magnesium) | 12 | 2, 8, 2 | Loses 2 e⁻ | Mg²⁺ |
| Cl (Chlorine) | 17 | 2, 8, 7 | Gains 1 e⁻ | Cl⁻ |
| O (Oxygen) | 8 | 2, 6 | Gains 2 e⁻ | O²⁻ |
Lattice Enthalpy
Lattice enthalpy is a critical concept in understanding the strength of ionic bonds. It is directly related to the force of attraction between ions and helps determine the physical properties of ionic solids. Compounds with high lattice enthalpy often show great thermal stability and resist physical deformation. Hence, it becomes an essential factor in designing heat-resistant materials, ceramics, and electrolytes in batteries. The magnitude of lattice enthalpy also helps predict solubility trends, bond strengths, and stability of ionic compounds in different environments.
Lattice enthalpy is the energy required to completely separate one mole of solid ionic compound into gaseous ions. Greater lattice enthalpy implies stronger ionic bonding and higher stability of the compound.
Example:
Lattice enthalpy of NaCl = −788 kJ mol⁻¹
Bond Parameters
Ionic bonding affects several measurable parameters:
| Bond Parameter | Description | Importance |
| Bond Length | Distance between nuclei of two bonded atoms | Determines stability |
| Bond Angle | Angle between bonds around central atom | Structural understanding |
| Bond Energy | Energy required to break a bond | Indicates bond strength |
| Van der Waals Radius | Overall size of atoms in non-bonded condition | Used in molecular geometry |
Properties of Ionic Compounds
Ionic compounds display characteristic physical and chemical properties due to the strong electrostatic interactions between their ions. These properties are not only important academically but are also significantly relevant in practical applications. For example, their high melting points make them useful in constructing heat-resistant linings, while their solubility in water enables their use in biological systems and medical formulations. These properties are also widely tested in competitive exams, making them crucial for conceptual clarity.
- High melting and boiling points
- Soluble in polar solvents like water
- Conduct electricity in molten or aqueous state
- Form crystalline solids
Key Characteristics of Ionic Compounds
1. High Melting and Boiling Points
Ionic compounds have strong electrostatic forces between ions. A large amount of energy is needed to break these bonds, which results in high melting and boiling points.
2. Soluble in Water
Water is a polar solvent and pulls ions apart easily. Hence, most ionic compounds are soluble in water.
3. Conduct Electricity in Molten or Aqueous State
In solid state, ions cannot move → no conduction
In molten/aqueous state, ions are free → conduct electricity
Tip:
Questions about electricity conduction are frequently asked in board exams. Students must specify the state of matter while answering.
Example with Full Explanation: Magnesium Oxide (MgO)
Electronic Configuration:
- Mg → 2, 8, 2 → loses 2 e⁻ → Mg²⁺
- O → 2, 6 → gains 2 e⁻ → O²⁻
Bond Formation:
Mg²⁺ and O²⁻ attract each other strongly → forms MgO, an ionic compound.
Important Exam Note:
This reaction is also used to explain valency and octet rule, therefore it is often used in exam questions.
Based Memory Trick
To remember trends easily:
“Metals Lose, Non-Metals Choose”
Metals lose electrons and become positive ions.
Non-metals choose to gain electrons and become negative ions.
FAQs
Q1: What is an ionic or electrovalent bond?
It is a chemical bond formed by the complete transfer of electrons from one atom to another, resulting in cations and anions.
Q2: Why do ionic compounds have high melting points?
Ionic compounds have strong electrostatic forces between ions, requiring high energy to break them.
Q3: What is lattice enthalpy?
Lattice enthalpy is the energy required to separate one mole of solid ionic compound into gaseous ions.
Q4: Do ionic compounds conduct electricity?
Yes, but only in molten or aqueous state when ions are free to move.
Conclusion
The concept of ionic or electrovalent bonding is crucial for understanding the structure and properties of numerous compounds. It beautifully explains how electron transfer leads to the formation of stable ions that create a strong crystalline lattice. From sodium chloride to calcium oxide, ionic bonding governs the behavior of many essential chemical substances. Mastering this topic forms the foundation for advanced concepts such as lattice energy, periodic trends, molecular geometry, and chemical reactivity, making it highly important in Class 11 Chemistry as well as competitive exams.





Get Social