Alkanes are the simplest and most fundamental class of hydrocarbons. They contain only carbon and hydrogen atoms connected through single covalent bonds. These molecules are also called saturated hydrocarbons because no more hydrogen atoms can be added without breaking the carbon framework. Alkanes form a major component of petroleum and natural gas and serve as the backbone for learning reaction mechanisms, structural behaviour, and the chemistry of more complex organic molecules.
General Characteristics of Alkanes
Alkanes share several common structural and chemical features:
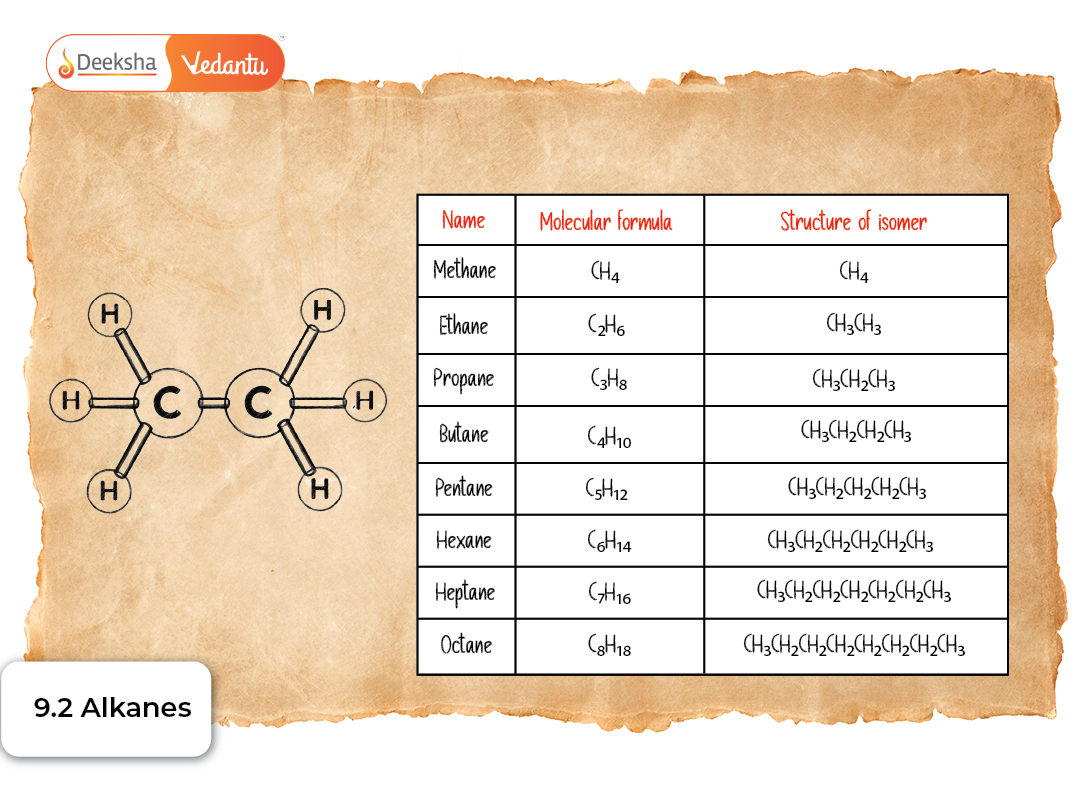
- They follow the general molecular formula CₙH₂ₙ₊₂.
- All carbon atoms are sp³ hybridised.
- The geometry around every carbon atom is tetrahedral with a bond angle of about 109.5°.
- They show very low chemical reactivity under normal conditions.
- Because of their low reactivity, they are often referred to as paraffins.
Naturally occurring alkanes are found in crude oil, petroleum gas, and natural gas. They are widely used as domestic and industrial fuels.
Structure and Bonding in Alkanes
The carbon atoms in alkanes undergo sp³ hybridisation, producing four strong sigma bonds around each carbon. These bonds form a stable tetrahedral arrangement. The sigma bonds allow free rotation, giving rise to multiple spatial arrangements called conformations.
Conformations of Alkanes
Rotation around C–C single bonds results in different conformations.
Conformations of Ethane
Ethane shows two extreme conformations:
- Staggered conformation: Hydrogens are positioned as far apart as possible; this is the most stable form.
- Eclipsed conformation: Hydrogens overlap when viewed along the carbon–carbon bond; this form has higher energy because of torsional strain.
Ethane continuously rotates between these conformations, but the staggered structure is energetically favoured.
Nomenclature of Alkanes
Alkanes are named using IUPAC rules. The name is based on the longest continuous carbon chain, which is selected as the parent chain.
Examples of Straight-Chain Alkanes
| Carbon Count | Name | Formula |
| 1 | Methane | CH₄ |
| 2 | Ethane | C₂H₆ |
| 3 | Propane | C₃H₈ |
| 4 | Butane | C₄H₁₀ |
| 5 | Pentane | C₅H₁₂ |
Branched alkanes are named by identifying substituents attached to the main chain, numbering the longest chain, and placing substituent names with their positions.
Preparation of Alkanes
Alkanes can be prepared through several laboratory and industrial methods.
1. From Unsaturated Hydrocarbons (Hydrogenation)
Hydrogenation involves the addition of hydrogen to alkenes or alkynes in the presence of finely divided metal catalysts such as Ni, Pt, or Pd. This process converts unsaturated hydrocarbons into saturated alkanes.
Principle:
- The double or triple bond of the unsaturated hydrocarbon undergoes addition of H₂.
- The metal catalyst weakens the π-bond, allowing sigma bond formation.
Example:
- CH₂=CH₂ + H₂ → CH₃–CH₃ (Ethene to ethane)
Important points:
- Used industrially in hydrogenation of vegetable oils.
- Yields are high and reactions proceed smoothly.
- Selectivity depends on catalyst type.
2. From Alkyl Halides
Alkyl halides can be reduced to alkanes by several reagents:
- H₂/Pt or H₂/Ni catalysts
- Zn/HCl (a mild, convenient reducing system)
- LiAlH₄ (highly effective but expensive)
General Reaction: R–X + H₂ → R–H + HX
Mechanistic insight:
- In catalytic reduction, hydrogen atoms adsorb on the metal surface.
- The alkyl halide also adsorbs and undergoes bond cleavage.
- Hydrogen attaches to the carbon skeleton, forming an alkane.
Limitations:
- Tertiary halides may produce rearranged products.
- Fluoroalkanes are less reactive toward reduction.
3. Wurtz Reaction
The Wurtz reaction is one of the earliest known methods for forming C–C bonds.
Reaction: 2R–X + 2Na → R–R + 2NaX
Mechanism (Simplified):
- Sodium metal donates electrons, generating alkyl radicals.
- Two radicals combine to form a new carbon–carbon bond.
Applications:
- Useful for preparing symmetrical higher alkanes.
Limitations:
- Ineffective for preparing unsymmetrical alkanes.
- Side‑reactions such as elimination may occur.
- Works best with primary alkyl halides.
4. Kolbe Electrolysis
Kolbe electrolysis involves the electrolysis of aqueous sodium or potassium salts of carboxylic acids.
General Reaction: 2R–COO⁻ → R–R + 2CO₂ + 2e⁻
Principle:
- At the anode, carboxylate ions undergo oxidative decarboxylation.
- The carbon radicals formed a couple to produce alkanes.
Features:
- Produces alkanes containing even number of carbon atoms.
- Works only for symmetrical alkanes.
- Useful in understanding radical coupling processes.
5. Decarboxylation (Soda-Lime Reaction)
Soda-lime decarboxylation is a traditional laboratory method.
Reaction: R–COONa + NaOH (CaO) → RH + Na₂CO₃
Principle:
- Heating the sodium salt of a carboxylic acid with soda‑lime removes CO₂.
- The remaining carbon fragment becomes an alkane.
Important notes:
- The product alkane has one fewer carbon atom.
- CaO prevents the mixture from fusing and increases efficiency.
- Industrial relevance is limited but academically important.
6. Corey–House Synthesis
One of the most elegant methods for forming higher alkanes.
General Reaction: R₂CuLi + R′–X → R–R′ + RLi + CuX
Mechanism (Simplified):
- Lithium dialkyl cuprate (Gilman reagent) transfers an alkyl group.
- The alkyl halide undergoes nucleophilic substitution.
Advantages:
- Produces unsymmetrical alkanes effectively.
- Works with a variety of alkyl halides.
Limitations:
- Requires organolithium reagents (moisture-sensitive).
- Not used in basic school laboratories due to high reactivity.
Summary Table for Quick Revision:
| Method | Reactants | Product | Key Feature |
| Hydrogenation | Alkenes/Alkynes + H₂ | Alkanes | Uses metal catalyst |
| Reduction | Alkyl halide + H₂/Zn–HCl | Alkane | Mild, high-yield method |
| Wurtz | Alkyl halides + Na | Symmetrical alkane | Radical coupling |
| Kolbe | Sodium salt of acid | Symmetrical alkane | Electrolytic decarboxylation |
| Decarboxylation | Sodium salt + NaOH/CaO | Alkane | One-carbon shorter product |
| Corey–House | R₂CuLi + R′–X | Unsymmetrical alkane | Highly selective C–C coupling |
Physical Properties of Alkanes
Alkanes display the following trends:
1. Physical State
- C₁ to C₄: Gases
- C₅ to C₁₇: Liquids
- Above C₁₇: Waxy solids
2. Solubility
- Insoluble in water due to non-polar nature
- Soluble in non-polar organic solvents
3. Density
Alkanes are lighter than water and float if mixed.
4. Boiling and Melting Points
- Increase with molecular weight
- Branched alkanes have lower boiling points than straight-chain isomers
5. Odour and Appearance
Lower alkanes are odorless while higher alkanes may have faint smells.
Chemical Properties of Alkanes
Despite being considered chemically inert, alkanes participate in several significant reactions under suitable conditions. Their reactivity is mainly due to C–H and C–C sigma bonds, which require considerable energy to break. However, when activated by heat, light, or catalysts, alkanes undergo transformations that are important industrially as well as academically.
1. Combustion
Combustion is the most prominent reaction of alkanes. In the presence of excess oxygen, alkanes undergo complete combustion to produce carbon dioxide, water, and large amounts of heat. This exothermic reaction is the basis for the use of alkanes as fuels.
- CH₄ + 2O₂ → CO₂ + 2H₂O
Features of combustion:
- Highly exothermic
- Produces a blue flame for smaller alkanes
- Used in LPG, CNG, petrol, and diesel engines
Incomplete combustion: When oxygen supply is limited, alkanes may form carbon monoxide or soot:
- 2CH₄ + 3O₂ → 2CO + 4H₂O
- CH₄ → C (soot) + 2H₂
Incomplete combustion is hazardous because CO is poisonous.
2. Controlled Oxidation
With specific metal catalysts, alkanes undergo partial oxidation to give valuable products:
- Alcohols (Cu catalyst at 523 K)
- Aldehydes (MoO₃ catalyst)
- Carboxylic acids (further oxidation)
For example, oxidation of propane may yield:
- CH₃–CH₂–CH₃ → CH₃–CH₂–OH (propanol)
- CH₃–CH₂–CH₃ → CH₃–CHO (acetaldehyde)
Industrial processes (like the production of methanol or acetic acid) rely heavily on controlled alkane oxidation.
3. Pyrolysis (Cracking)
Pyrolysis involves breaking long‑chain alkanes into smaller alkanes, alkenes, or hydrogen by heating to high temperatures (typically 773–1273 K).
Example:
- C₁₀H₂₂ → C₅H₁₂ + C₅H₁₀
Types of cracking:
- Thermal cracking: purely heat-based
- Catalytic cracking: uses zeolites or Al₂O₃ catalysts; more selective
This process is fundamental in the petroleum industry to convert heavy fractions into usable fuels.
4. Aromatisation
Straight‑chain alkanes containing six or more carbon atoms can be converted to aromatic hydrocarbons when heated at high temperatures in the presence of catalysts like V₂O₅ or MoO₃.
Example:
- n-hexane → benzene + hydrogen
Why aromatisation occurs:
- Aromatic compounds are thermodynamically stable
- Dehydrogenation helps introduce unsaturation and ring formation
Aromatisation is essential in the petrochemical industry for the production of benzene, toluene, and xylene.
5. Halogenation (Free Radical Substitution)
Halogenation is the most important chemical reaction of alkanes. In the presence of UV light or high temperature, alkanes react with chlorine or bromine to form haloalkanes.
The mechanism follows a free radical chain reaction involving three steps:
Initiation
The halogen molecule splits into free radicals due to homolytic cleavage.
- Cl₂ → 2Cl·
Propagation
Radicals attack alkanes to form new radicals:
- CH₄ + Cl· → CH₃· + HCl
- CH₃· + Cl₂ → CH₃Cl + Cl·
This creates a chain process where radicals continuously form and react.
Termination
Radicals combine to end the chain reaction:
- Cl· + Cl· → Cl₂
- CH₃· + CH₃· → C₂H₆
Key features:
- Halogenation is random; multiple substitution products are common
- Bromination is more selective than chlorination because it is less reactive
6. Nitration
At high temperatures (around 673 K), alkanes react with nitric acid vapours to give nitroalkanes.
- CH₄ + HNO₃ → CH₃NO₂ + H₂O
This is called vapour-phase nitration and is important in preparing nitromethane and nitroethane.
Characteristics:
- Harsh reaction conditions
- Not highly selective
- Used for industrial synthesis of organic nitro compounds
7. Sulphonation
Alkanes react with fuming sulphuric acid (H₂SO₄ + SO₃) to form alkyl sulphonic acids.
- RH + H₂SO₄ → R–SO₃H + H₂
Features:
- Occurs at high temperatures
- Most effective with higher alkanes
- Sulphonic acids are important intermediates in detergent and surfactant production
Uses of Alkanes
- Fuels such as LPG, petrol, diesel, and kerosene
- Industrial solvents
- Raw materials for alcohols, detergents, and polymers
- Components of natural gas and petroleum
FAQs
Q1. Why are alkanes called saturated hydrocarbons?
Because they contain only single bonds and cannot add more hydrogen atoms without breaking the carbon skeleton.
Q2. Why are alkanes less reactive?
Their strong sigma bonds require a lot of energy to break, making them chemically stable.
Q3. What makes staggered conformation more stable?
It minimises repulsion between hydrogen atoms, lowering energy.
Q4. Why are alkanes important as fuels?
They release a large amount of heat on combustion.
Q5. What type of reaction occurs during halogenation?
Free radical substitution involving initiation, propagation, and termination.
Conclusion
Alkanes form the base of organic chemistry due to their structural simplicity, predictable behaviour, and industrial importance. Understanding their structure, preparation methods, and chemical properties builds a strong foundation for mastering more complex hydrocarbons. At Deeksha Vedantu, concepts are explained clearly with emphasis on application and exam readiness.





Get Social