The Valence Shell Electron Pair Repulsion (VSEPR) theory is one of the most fundamental concepts in chemical bonding. It helps in predicting the three-dimensional shape of molecules based on the repulsion between electron pairs in the valence shell of atoms. According to this theory, electron pairs — whether involved in bonding or present as lone pairs — always try to stay as far apart as possible. By analyzing these repulsions, chemists can determine the geometry of molecules and understand how structure influences reactivity and polarity. VSEPR theory becomes especially important while visualizing molecules in three-dimensional space, which is essential for organic reaction mechanisms and hybridisation.
Main Postulates of VSEPR Theory
The theory is based on a few simple but powerful assumptions about electron arrangements:
- The shape of a molecule depends on the total number of valence shell electron pairs around the central atom.
- Both bonding pairs and lone pairs repel each other and try to minimize repulsion by adjusting their positions.
- The valence shell is treated as a spherical region containing electron pairs.
- Multiple bonds (double or triple) are treated as a single electron domain.
- Lone pairs occupy more space than bonding pairs, causing distortion from ideal geometry.
- Molecules attempt to achieve maximum stability by minimizing repulsion.
These postulates allow the prediction of geometrical shapes even before experimentally measuring bond angles or lengths.
Types of Electron Pair Repulsions
The strength of repulsion between electron pairs follows this order:
Lone pair–Lone pair > Lone pair–Bond pair > Bond pair–Bond pair
This trend explains why certain molecules deviate from ideal angles. For example, the greater the number of lone pairs, the more compressed the bond angles become. This concept is used widely for structural prediction, especially for molecules like NH₃, H₂O, and SF₄.
Geometry of Molecules without Lone Pairs
When the central atom has no lone pairs, the shapes are ideal and symmetrical. These geometries form the backbone of molecular structure in chemistry:
| No. of Electron Pairs | Arrangement | Molecular Geometry | Examples |
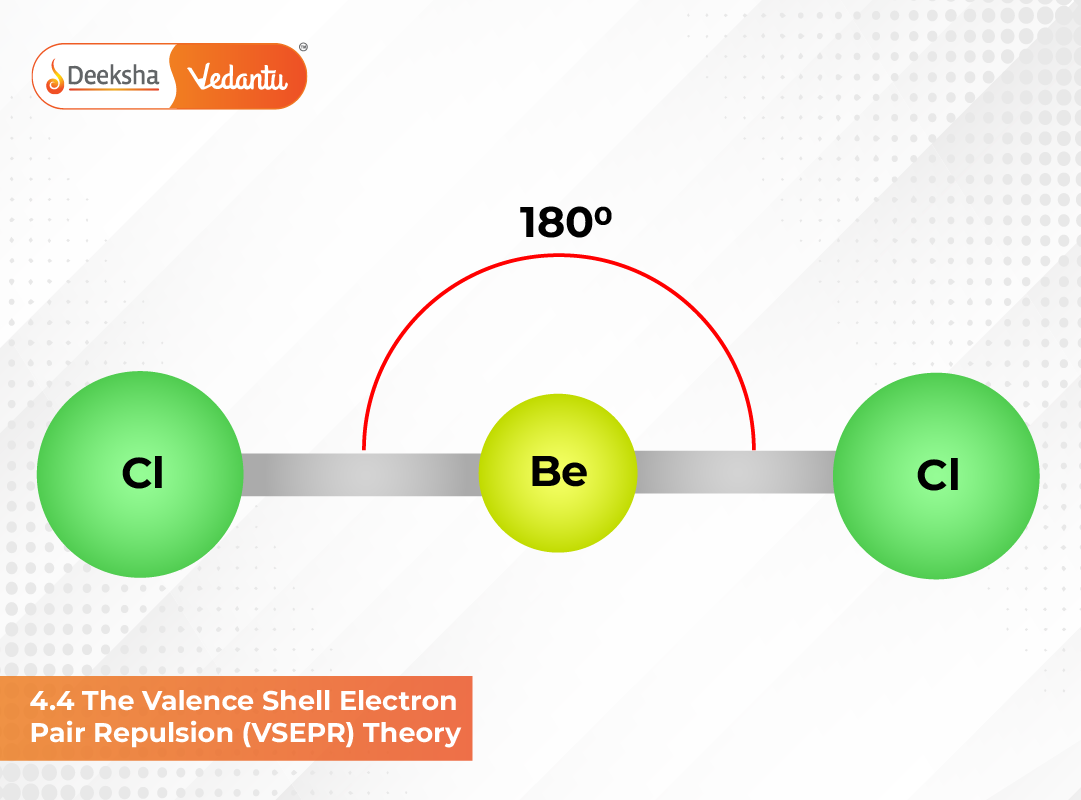
| 2 | 180° | Linear | BeCl₂, HgCl₂ |
| 3 | 120° | Trigonal planar | BF₃ |
| 4 | 109.5° | Tetrahedral | CH₄, NH₄⁺ |
| 5 | 120°, 90° | Trigonal bipyramidal | PCl₅ |
| 6 | 90° | Octahedral | SF₆ |
Text Explanation: The geometry depends entirely on electron pair repulsion. A larger number of electron domains leads to complex arrangements, balancing repulsion in three dimensions.
Geometry of Molecules with Lone Pairs
In such molecules, lone pairs cause distortion, changing bond angles and geometry:
| Molecule Type | Bond Pairs | Lone Pairs | Shape | Example |
| AB₂E | 2 | 1 | Bent | SO₂ |
| AB₃E | 3 | 1 | Trigonal pyramidal | NH₃ |
| AB₂E₂ | 2 | 2 | Bent (V-shaped) | H₂O |
| AB₄E | 4 | 1 | See-saw | SF₄ |
| AB₃E₂ | 3 | 2 | T-shaped | ClF₃ |
| AB₅E | 5 | 1 | Square pyramidal | BrF₅ |
| AB₄E₂ | 4 | 2 | Square planar | XeF₄ |
Why shapes change: Lone pairs repel more strongly than bonding pairs because they are localized closer to the nucleus. This causes bond angles to shrink compared to ideal values.
Ball-and-Stick Model
Imagine the central atom at the center of a sphere with bonding pairs and lone pairs positioned around it. The more lone pairs present, the more the molecule deviates from the ideal geometry, leading to bent, pyramidal, T-shaped, or square planar structures.
Examples of Bond Angle Distortion Due to Lone Pair Repulsion
- CH₄ (Methane): 109.5° (ideal tetrahedral, no lone pair)
- NH₃ (Ammonia): 107° (one lone pair → trigonal pyramidal)
- H₂O (Water): 104.5° (two lone pairs → bent shape)
This progressive reduction in bond angle clearly proves that lone pair repulsion > bond pair repulsion.
Real-life Applications of VSEPR Theory
VSEPR theory is widely used in various scientific fields:
- Molecular structure prediction in organic chemistry
- Drug design and pharmaceutical research
- Protein folding and enzyme action analysis
- Determination of polarity and physical properties
- Molecular modeling in computational chemistry
- Designing pesticides and industrial chemicals
Understanding molecular geometry is essential for predicting reactivity, acidity/basicity, boiling point, solubility, and dipole moment of compounds.
Common Mistakes Students Should Avoid
- Confusing lone pairs as bonding domains
- Ignoring double/triple bonds treated as one domain
- Forgetting that bond angles shrink with lone pairs
- Assuming geometry only depends on bond pairs
Such errors often lead to incorrect molecular shapes in exams. Using domain counting and VSEPR table reference helps avoid mistakes.
FAQs
Q1: What does VSEPR theory explain?
It predicts the 3D molecular shape based on repulsion between bonding and lone electron pairs.
Q2: Which molecule shows perfect tetrahedral geometry?
Methane (CH₄) has a perfectly tetrahedral arrangement.
Q3: Why do lone pairs distort ideal geometry?
Lone pairs occupy more space and repel bond pairs strongly, reducing bond angles.
Q4: How does VSEPR relate to hybridisation?
The molecular geometry predicted by VSEPR helps determine the hybridisation type (sp, sp², sp³, etc.).
Conclusion
The VSEPR theory is a simple yet powerful model that explains molecular shapes using electron pair repulsion. It connects deeply with hybridisation, bond angles, polarity, and chemical reactivity. By combining theory with NCERT tables and real-world examples, students can develop strong conceptual understanding essential for Class 11 Chemistry, NEET, JEE, and advanced chemical studies. Mastery of VSEPR theory builds the foundation for organic reaction mechanisms and molecular orbital theory in later chapters.






Get Social