Valence Bond Theory (VBT) was introduced to explain how atoms bond together through orbital overlap to form stable molecules. While earlier theories like the Lewis and VSEPR models helped understand basic bonding and shapes of molecules, they could not explain the variation in bond energies and bond lengths accurately. VBT provides a deeper and more scientific understanding of how chemical bonds form based on atomic orbital interactions and electron pairing.
This theory was first proposed by Heitler and London, and later refined by Linus Pauling. According to VBT, atoms approach one another in such a way that their atomic orbitals overlap. When this overlap is efficient, a shared electron pair is formed between the atoms, and a covalent bond is established. The strength of the bond depends on the extent of orbital overlap — greater the overlap, stronger the bond. NCERT emphasizes that VBT laid the foundation for understanding hybridisation and modern bonding theories.
Forces During Bond Formation
When two atoms begin to approach each other, two types of forces come into play:
- Attractive forces between the nucleus of one atom and the electrons of the other atom.
- Repulsive forces between electrons of both atoms and between the two nuclei.
Initially, attractive forces dominate, allowing the atoms to move closer. But when they come too close, repulsive forces increase rapidly. A chemical bond is stable only when minimum energy is achieved. The distance at which this minimum energy occurs is known as the bond length of the molecule.
Example: Formation of H₂ Molecule (NCERT Style)
When two hydrogen atoms approach each other, their 1s orbitals overlap partially. As they reach the optimum distance, the most stable bond is formed. The energy released during this process is called bond enthalpy, which indicates the bond strength.
Reaction:
H(g) + H(g) → H₂(g)
Bond enthalpy = 435.8 kJ mol⁻¹
Thus, energy is released when a bond is formed and energy must be absorbed to break a bond. The graph of potential energy vs. internuclear distance shows the ideal point of bond formation.
Orbital Overlap Concept
According to VBT, the overlap of atomic orbitals leads to bond formation. Greater overlap means stronger bonds. This concept explains both the strength and directionality of covalent bonds. NCERT highlights that orbital overlap also helps explain why specific molecules have specific shapes.
Types of Orbital Overlaps:
- Positive overlap (in-phase): Occurs when orbitals align properly → Strong bond formation.
- Negative overlap (out-of-phase): Incorrect alignment → No bond forms.
- Zero overlap: Atoms remain unbonded due to incorrect orientation.
Directional Properties of Bonds
Valence Bond Theory explains why molecules like CH₄, NH₃, and H₂O have different shapes even though they all involve the central atom forming bonds. This is due to:
- Directional overlap of orbitals
- Hybridisation of orbitals for stability
- Lone pair–bond pair repulsion (explained in VSEPR theory)
VBT complements VSEPR by explaining why bonds are oriented in specific directions and how orbital geometry affects molecular shape.
Overlapping of Atomic Orbitals
Bond formation depends on the following factors:
- Orientation of orbitals
- Phase alignment (positive/negative)
- Distance between atoms
Based on these parameters, covalent bonds are classified into two main types:
Types of Covalent Bonds
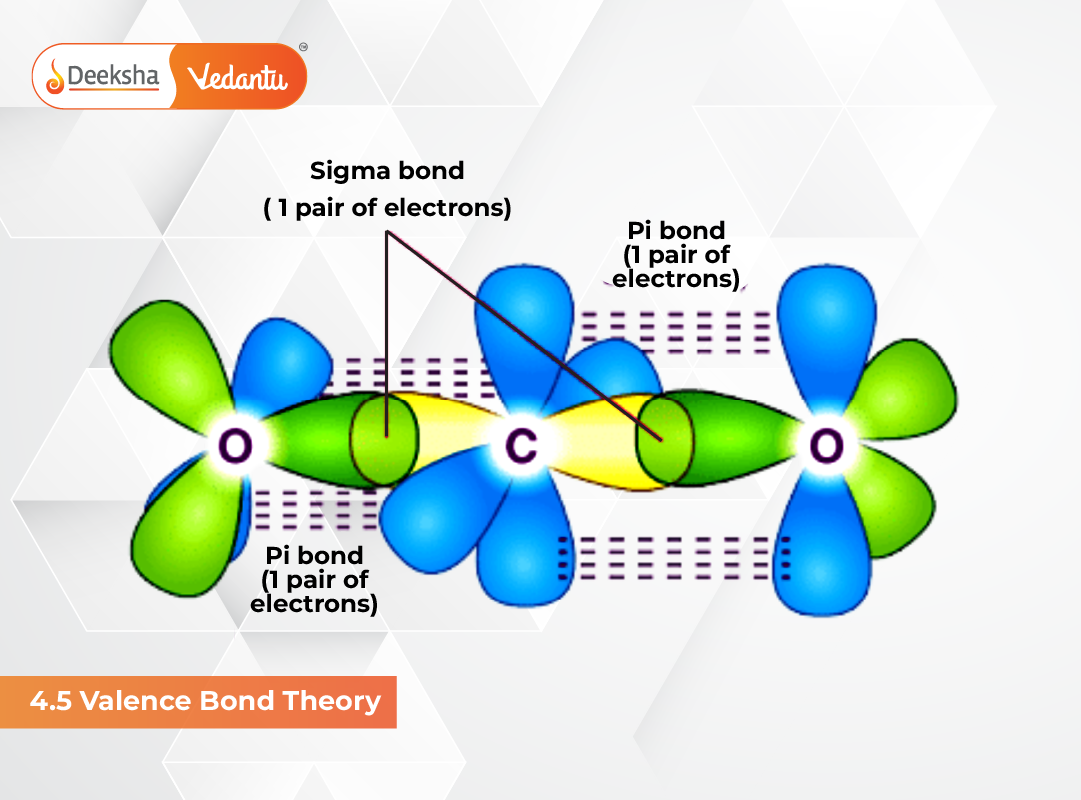
1. Sigma (σ) Bond
Formed by head-on (end-to-end) overlap of orbitals. It is the strongest type of covalent bond and allows free rotation. Types include:
- s–s overlap → simplest bonding
- s–p overlap → common in organic compounds
- p–p overlap → common in double-bonded systems
2. Pi (π) Bond
Formed by sidewise overlap of p-orbitals. It is weaker than sigma bonds and restricts rotation around the bond axis.
Examples:
- Ethene (C₂H₄): 1 sigma + 1 pi bond
- Nitrogen (N₂): 1 sigma + 2 pi bonds → triple bond
Strength of Sigma and Pi Bonds
- Sigma bond → greater overlap → stronger
- Pi bond → less overlap → weaker
- Multiple bonds shorten bond length and increase rigidity
NCERT Notes: Sigma bonds decide bond orientation, while pi bonds influence reactivity and restrict rotation.
Importance of Valence Bond Theory
- Explains bond strength and bond length
- Gives direction to covalent bonds
- Helps understand molecular geometry
- Connects to hybridisation and VSEPR theory
- Basis of chemical reactivity and polarity of molecules
FAQs
Q1: What does Valence Bond Theory explain?
It explains that covalent bonds form due to the overlap of atomic orbitals and sharing of electron pairs.
Q2: Why are sigma bonds stronger than pi bonds?
They are formed by head-on overlap, which results in greater electron density between the nuclei.
Q3: Who developed Valence Bond Theory?
It was proposed by Heitler and London, later refined by Linus Pauling.
Conclusion
Valence Bond Theory (VBT) marked a significant advancement in chemical bonding theories by scientifically explaining how covalent bonds form through orbital overlap. It not only explains bond strength and molecular geometry but also acts as the foundation for hybridisation and modern bonding theories. VBT is essential for mastering Class 11 Chemistry and plays a major role in NEET and JEE examinations where questions often test its concepts through numerical problems and molecular analysis.





Get Social