Organic chemistry is a vast and diverse branch of science that studies millions of carbon-containing compounds found in living organisms, fuels, medicines, plastics, fibres, and countless industrial materials. Because there are so many organic compounds, understanding their structures becomes essential. Structural representations act as a universal language that allows chemists around the world to communicate clearly about molecular architecture, bonding, geometry, and function. This makes the topic of structural representations foundational for every student beginning their journey in organic chemistry.
NCERT Class 11 Chapter 8.3 introduces students to the various ways organic compounds can be represented graphically and symbolically. At Deeksha Vedantu, students learn how to interpret, convert, and construct these structures through practical exercises, visual tools, and systematic explanation. This ensures they not only memorize these formats but also understand the reasoning and logic behind each representation.
Importance of Structural Representations
Structural representations are significant because organic molecules cannot be fully understood through molecular formulas alone. A molecular formula only tells you the number of atoms present-for example, C₄H₁₀-but it cannot tell you whether the compound is butane or isobutane. These compounds differ in physical properties such as boiling point, melting point, and stability, which only become clear when the structure is shown.
Structural representations enable students to:
- Identify and differentiate between isomers
- Recognize functional groups and substituent positions
- Visualize three-dimensional molecular geometry
- Predict chemical reactivity and possible reaction pathways
- Understand molecular polarity and intermolecular forces
- Interpret reaction mechanisms and electron movement diagrams
A strong understanding of structure is essential for advanced organic topics like stereochemistry, conformational analysis, spectroscopy, and synthetic pathway design.
Types of Structural Representations
Organic compounds can be represented in several ways, each designed for a specific purpose. Some formats emphasize connectivity, while others highlight geometry or three-dimensional orientation. Becoming fluent in all formats helps students quickly interpret textbook materials and solve competitive exam questions.
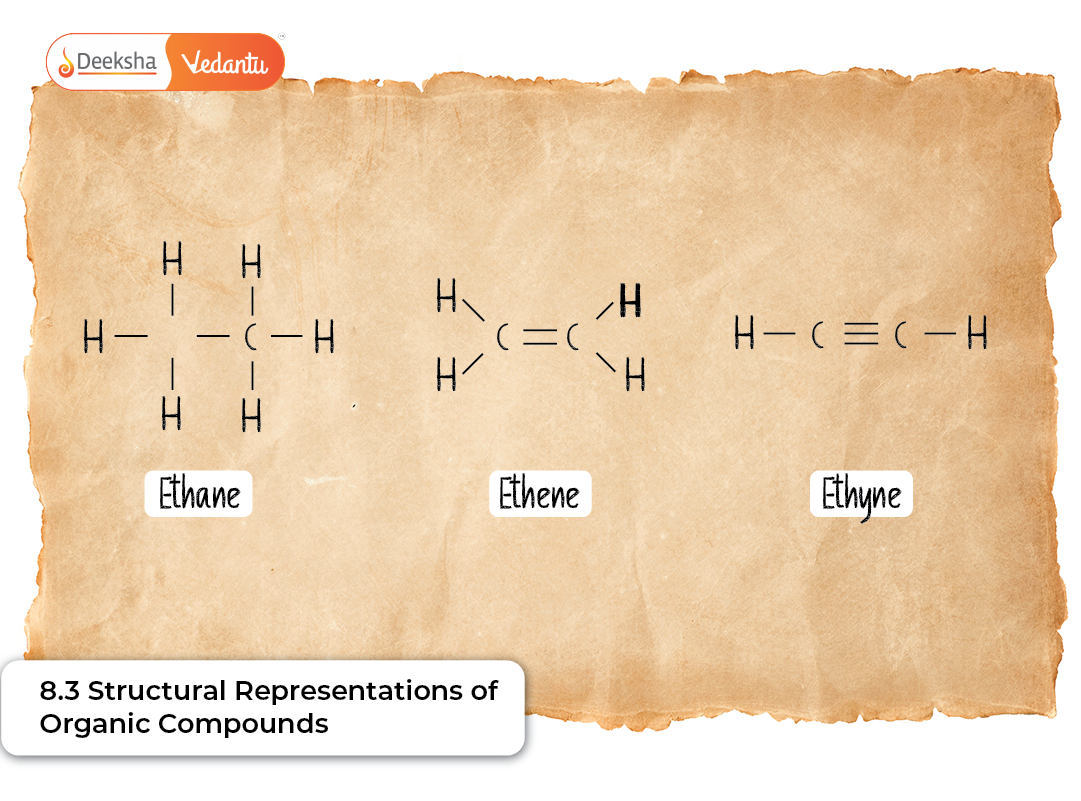
Complete Structural Formula (Lewis Structure)
The complete structural formula, often called the Lewis structure, gives the most detailed representation of a molecule. It displays:
- Every atom in the molecule
- All bonds-single, double, or triple
- Lone pairs of electrons (when needed)
This structure is extremely important for beginners because it shows all bonding and helps visualize how atoms are connected. It also helps students understand concepts such as bond polarity, bond order, and resonance.
Example: Hexane: CH₃–CH₂–CH₂–CH₂–CH₂–CH₃
This makes it clear that hexane is a straight-chain hydrocarbon with no branching.
Condensed Structural Formula
Condensed structural formulas offer a shorter, more compact version while still showing the order of atoms. Instead of drawing bonds, atoms bonded to a carbon are written next to it.
Example: Hexane (condensed) CH₃CH₂CH₂CH₂CH₂CH₃
This saves time and space while still conveying the connectivity of atoms. Condensed formulas are used frequently in textbooks, examinations, and research papers.
Bond-Line (Skeletal) Structure
Bond-line or skeletal structures are the most widely used method for representing organic compounds. In this format:
- Carbon atoms are implied at the ends and intersections of lines
- Hydrogen atoms bonded to carbons are not shown
- Heteroatoms (like O, N, Cl, S) must be drawn explicitly
- Double and triple bonds are drawn as multiple lines
Example: Drawing hexane as a zig-zag bond-line diagram instantly highlights its chain structure.
Skeletal structures are extremely useful for complex molecules as they reduce visual crowding, simplify large frameworks, and highlight functional groups clearly.
Three-Dimensional (3D) Representation
3D structural representations are essential for molecules whose spatial arrangement affects their behaviour and reactivity. These diagrams use:
- Solid wedges for bonds projecting toward the viewer
- Dashed wedges for bonds going behind the plane
- Straight lines for bonds in the plane
3D models help students understand concepts like chirality, stereoisomerism, and molecular geometry.
Example: Chandelling methane’s 3D tetrahedral shape becomes clear with wedge-and-dash notation.
Expanded Explanation of Each Representation
Why Complete Structural Formulas Are Essential
Complete structures help students avoid misconceptions by showing:
- Precise connectivity
- Exact bond types
- Functional group placement
- Lone pairs relevant to reactivity
These structures allow for accurate resonance structure drawing and mechanism analysis.
Benefits of Condensed Structural Formulas
Condensed formulas help students:
- Write long chains efficiently
- Identify repeating units such as –CH₂–
- Recognize substituents easily
- Transition toward skeletal structures
They act as a step between detailed structures and simplified formats.
Why Skeletal Structures Dominate Organic Chemistry
Chemists prefer skeletal structures because:
- They eliminate unnecessary details
- They clearly display branching patterns
- They emphasize functional groups and ring systems
- They simplify large molecules such as steroids or terpenes
For competitive exams like JEE and NEET, skeletal structures save time and reduce errors.
Importance of 3D Representations
Three-dimensional models reveal:
- Spatial orientation of substituents
- Chirality (R/S configuration)
- Cis/trans or E/Z isomerism
- Stereoselectivity in reactions
These are vital for pharmaceuticals, where molecular shape determines biological response.
Converting Between Structural Forms
Students must master conversions because a single organic compound may appear in multiple representation styles depending on the context. Examinations, textbooks, and reference materials often switch between formats, and the ability to translate one structure into another ensures that students fully grasp connectivity, geometry, and the underlying logic of molecular construction.
Understanding conversions also strengthens skills in identifying isomers, predicting functional group behaviour, and interpreting reaction mechanisms. At Deeksha Vedantu, these conversions are taught through structured practice sessions, visual breakdowns, and guided examples.
Molecular Formula → Complete Structure
This step requires understanding valency rules, electron configuration, and typical bonding patterns. Students must determine how many bonds each atom can form, how carbon chains may be arranged, and where functional groups might be placed. For example, converting C₄H₁₀ requires deciding whether it forms a straight-chain or branched arrangement—leading to butane or isobutane.
Complete Structure → Condensed Formula
In this conversion, students rewrite the molecule by grouping atoms bonded to each carbon. It promotes clarity and compactness. For example, CH₃–CH₂–CH₂–CH₃ becomes CH₃CH₂CH₂CH₃. This step helps students recognize patterns like –CH₂– units and identify branching more easily.
Condensed Formula → Skeletal Structure
This step is crucial for representing complex molecules efficiently. Students remove explicit carbon symbols and implied hydrogens, replacing them with line intersections and endpoints. Functional groups are kept visible. This conversion enhances the ability to identify chain length, branching, ring systems, and unsaturation at a glance.
Skeletal Structure → 3D Representation
This final transition introduces spatial understanding. Students interpret bond angles and hybridisation to decide where to place wedges, dashes, or planar bonds. It is especially important for molecules with chiral centres or restricted rotation. The 3D form helps reveal stereochemistry, molecular orientation, and reactivity trends essential in advanced organic reactions.
At Deeksha Vedantu, students practice these conversions through worksheets, interactive diagrams, and conceptual drills.
Real-World Examples and Applications
Example 1: Glucose
Glucose can be shown as:
- A complete structure with six hydroxyl groups
- A condensed formula like C₆H₁₂O₆
- A 3D cyclic Haworth projection
These help explain energy production in biology.
Example 2: Aspirin
Aspirin’s structure includes:
- Aromatic ring
- Ester linkage
- Carboxylic acid group
Its skeletal structure highlights its pharmacological importance.
Example 3: Amino Acids
Amino acids are chiral and require 3D models to show stereochemistry. This explains protein folding and enzyme interactions.
Example 4: Hydrocarbons
Butane and isobutane show structural isomerism clearly through skeletal or condensed formulas.
Advanced Structural Features Students Should Know
- Resonance structures show delocalized electrons
- Formal charges clarify electron distribution
- Aromaticity symbols like circles in benzene help visualize delocalization
- Isomeric variations illustrate structural diversity
These concepts prepare students for Class 12 organic chemistry and competitive exam problem-solving.
FAQs
Q1. Why are structural representations important in organic chemistry?
They illustrate how atoms are connected, arranged, and influence properties and reactivity.
Q2. What is the difference between condensed and skeletal formulas?
Condensed formulas list atoms together, while skeletal diagrams reduce carbon bonds to lines.
Q3. Why do chemists prefer skeletal structures?
They are fast, simple, and focus attention on key structural elements.
Q4. When are 3D structural representations needed?
They are used for stereochemistry, chirality, and visualising spatial arrangements.
Q5. Do different representations show different information?
Yes. Each format highlights unique features of the molecule.
Conclusion
Structural representations are the foundation of organic chemistry learning. From detailed Lewis structures to compact condensed formulas, elegant skeletal diagrams, and precise 3D models, each format offers valuable scientific insight. Mastery of these forms empowers students to predict reactivity, solve mechanisms, interpret isomerism, and understand complex organic behaviour.
At Deeksha Vedantu, we strengthen students’ structural understanding through guided learning, visual tools, and continuous practice so they can confidently progress to stereochemistry, reaction mechanisms, and advanced organic transformations.






Get Social