The concept of spontaneity is central to understanding why certain processes occur naturally without any external influence, while others require continuous energy input. In thermodynamics, spontaneity determines the direction in which a chemical or physical process proceeds under given conditions. However, spontaneity does not imply speed – a spontaneous process can be extremely slow, such as the rusting of iron.
Meaning of Spontaneous Processes
A process is said to be spontaneous if it occurs naturally under given conditions without external intervention. It can proceed on its own once initiated. Conversely, non-spontaneous processes require continuous external energy to occur.
For example:
- Flow of water downhill occurs spontaneously, but upward flow requires energy (like pumping).
- The diffusion of gases is spontaneous, but compression of gases requires work.
Thermodynamics helps us predict whether a process is spontaneous or not by analyzing changes in energy and entropy.
(a) Is Decrease in Enthalpy (ΔH) a Criterion for Spontaneity?
At first glance, it might seem that a process accompanied by a decrease in enthalpy (ΔH < 0) – i.e., an exothermic reaction – should always be spontaneous. Indeed, many spontaneous reactions are exothermic, as energy is released to the surroundings.
Examples of Exothermic Spontaneous Reactions:
- ½ N₂(g) + 3/2 H₂(g) → NH₃(g); ΔrH° = –46.1 kJ mol⁻¹
- C(graphite) + O₂(g) → CO₂(g); ΔrH° = –393.5 kJ mol⁻¹
However, not all exothermic reactions are spontaneous, and not all spontaneous reactions are exothermic. For instance, melting of ice and vaporization of water are endothermic but occur spontaneously under certain conditions.
Therefore, while enthalpy change contributes to spontaneity, it cannot solely determine it.
(b) Entropy and Spontaneity
To fully understand spontaneity, we must consider entropy (S) – a measure of disorder or randomness in a system. A spontaneous process is often accompanied by an increase in total entropy of the system and surroundings.
Definition: Entropy is the degree of randomness or disorder in a system. Higher entropy means greater randomness.
Example: When two gases are allowed to mix, their molecules become more randomly distributed, increasing entropy.
For an isolated system, the second law of thermodynamics states that:
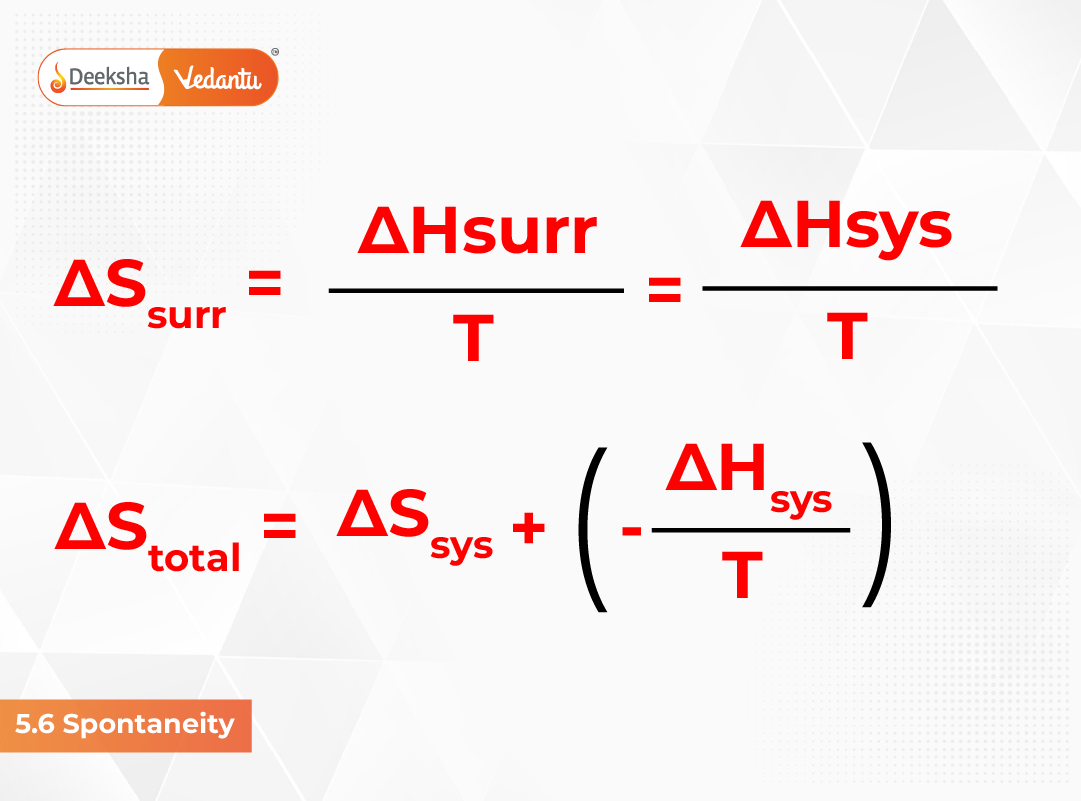
ΔSuniv = ΔSsys + ΔSsurr > 0
(For spontaneous processes)
Entropy and Phase Changes
- Solid → Liquid → Gas (Entropy increases)
- Gas → Liquid → Solid (Entropy decreases)
Thus, spontaneous processes often increase the entropy of the universe.
(c) Quantifying Entropy Change
The change in entropy for a reversible process is given by:
ΔS = qrev / T
Where:
- qrev = heat absorbed or released reversibly
- T = absolute temperature
For spontaneous reactions:
ΔStotal = ΔSsys + ΔSsurr > 0
If ΔStotal < 0, the process is non-spontaneous.
Example: In the diffusion of gases, entropy increases as gas particles spread evenly throughout the container.
(d) Gibbs Energy and Spontaneity
Since both energy (enthalpy) and entropy influence spontaneity, we define a new thermodynamic function called Gibbs free energy (G).
Definition:
G = H – TS
The change in Gibbs energy during a process is given by:
ΔG = ΔH – TΔS
This equation combines both enthalpy and entropy effects to predict spontaneity under constant temperature and pressure.
Criterion for Spontaneity
- If ΔG < 0, the process is spontaneous.
- If ΔG = 0, the system is at equilibrium.
- If ΔG > 0, the process is non-spontaneous.
This makes Gibbs free energy a powerful tool to evaluate spontaneity quantitatively.
(e) Relationship Between Enthalpy, Entropy, and Temperature
Temperature plays a significant role in determining spontaneity, especially for endothermic reactions.
Rearranging ΔG = ΔH – TΔS:
TΔS = ΔH (for equilibrium)
Hence, at higher temperatures, reactions with positive ΔS (increase in entropy) may become spontaneous even if ΔH is positive.
Example: Melting of ice at 0°C.
- ΔH = +6.0 kJ mol⁻¹
- ΔS = +22 J K⁻¹ mol⁻¹
At low temperatures, ΔG > 0 (non-spontaneous), but above 273 K, ΔG becomes negative, making melting spontaneous.
(f) Entropy and the Second Law of Thermodynamics
The Second Law of Thermodynamics states that the total entropy of an isolated system always increases for a spontaneous process. This law explains why heat flows from hot to cold bodies and why exothermic reactions tend to occur naturally.
ΔStotal = ΔSsys + ΔSsurr > 0
Thus, spontaneous changes in the universe are those that increase total entropy.
(g) Absolute Entropy and Third Law of Thermodynamics
The Third Law of Thermodynamics states that the entropy of a perfectly crystalline substance at absolute zero (0 K) is zero. This provides a reference point for measuring absolute entropy.
S = 0 at T = 0 K (for a perfect crystal)
As temperature increases, atomic vibrations cause an increase in entropy. Absolute entropy values can be experimentally determined by summing (qrev/T) increments from 0 K to 298 K.
Summary Table: Criteria for Spontaneity
| Parameter | Condition | Type of Process |
| ΔH < 0, ΔS > 0 | Always spontaneous | Exothermic and disorder increasing |
| ΔH > 0, ΔS < 0 | Never spontaneous | Endothermic and disorder decreasing |
| ΔH < 0, ΔS < 0 | Spontaneous at low T | Exothermic but ordering increases |
| ΔH > 0, ΔS > 0 | Spontaneous at high T | Endothermic but disorder increases |
FAQs
Q1. Does a spontaneous reaction mean it is fast?
No. Spontaneity only indicates the direction of a reaction, not its rate. Some spontaneous reactions, like rusting, occur slowly.
Q2. Can an endothermic reaction be spontaneous?
Yes, if the increase in entropy (ΔS) is large enough to make ΔG negative.
Q3. What is the importance of Gibbs free energy?
ΔG helps predict whether a reaction will proceed spontaneously under given conditions.
Q4. Why does entropy increase in spontaneous processes?
Because systems naturally evolve towards greater disorder or randomness.
Q5. How is the Third Law of Thermodynamics useful?
It provides a basis for calculating absolute entropies of substances at any temperature.
Conclusion
Spontaneity depends on both energy and disorder. While enthalpy change (ΔH) influences the heat exchange, entropy (ΔS) determines the direction of disorder. The combined effect is expressed through Gibbs free energy (ΔG = ΔH – TΔS), which serves as the definitive criterion for spontaneity. A solid understanding of these relationships allows students to analyze chemical processes and predict their natural feasibility under various conditions – an essential skill for mastering thermodynamics in NEET and JEE Chemistry.





Get Social