Importance of Chemistry
Chemistry is known as the central science because it connects physics, biology, and environmental sciences. It helps explain natural phenomena, develop new materials, design medicines, and understand biological processes. From the food we eat to the air we breathe, chemistry shapes every aspect of our lives. In academics, it lays the foundation for higher studies in medicine, engineering, and research.
Applications of Chemistry:
- Development of pharmaceuticals and polymers.
- Environmental protection through chemical analysis.
- Innovations in nanotechnology and energy storage.
- Understanding biological and molecular structures.
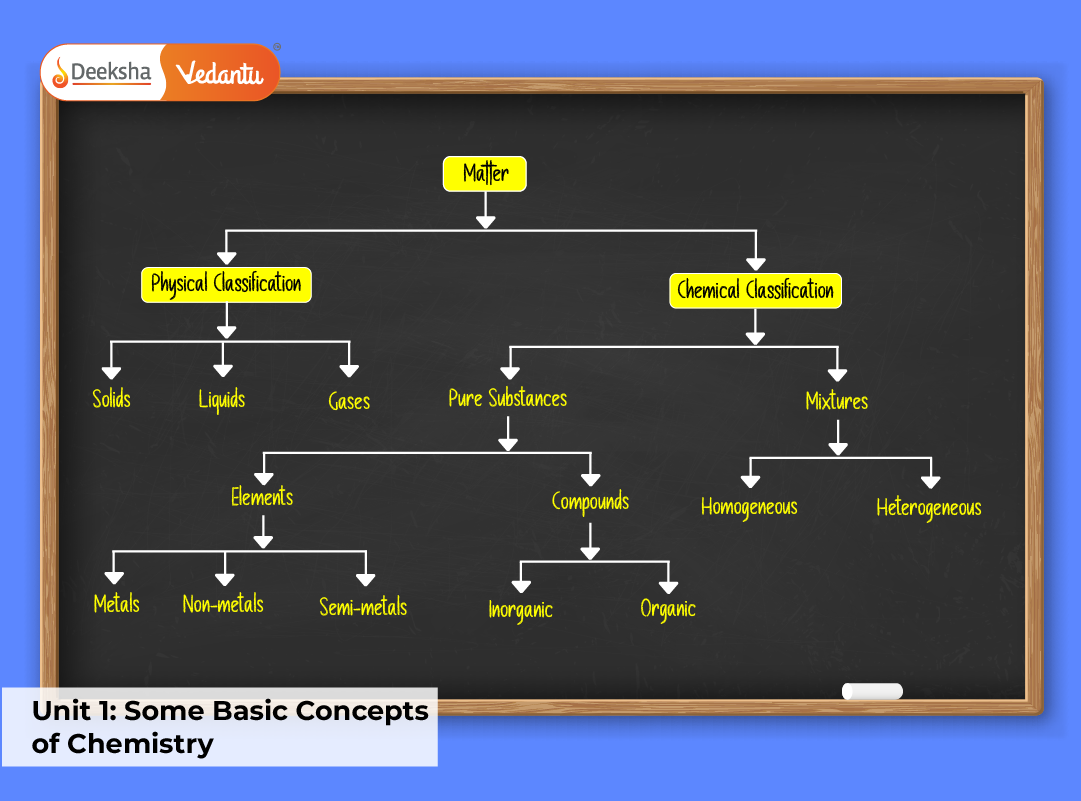
Nature of Matter
Matter is anything that has mass and occupies space. It exists in three primary states — solid, liquid, and gas — determined by the arrangement and motion of particles. Matter can also exist as plasma and Bose-Einstein condensates under extreme conditions.
Classification of Matter:
- Pure Substances: Elements and compounds with fixed composition (e.g., oxygen, water).
- Mixtures: Physical combinations of substances that can be separated by physical means.
Matter’s physical and chemical nature defines its behavior and interaction with energy.
Properties of Matter and Their Measurement
Properties of matter are of two types:
- Physical Properties: Measurable without changing chemical identity (e.g., color, density, melting point).
- Chemical Properties: Observed during chemical reactions (e.g., flammability, reactivity).
Measurement:
Quantitative measurement involves units and standards — the International System of Units (SI) is most commonly used. For instance:
- Length → metre (m)
- Mass → kilogram (kg)
- Time → second (s)
- Temperature → kelvin (K)
- Amount of substance → mole (mol)
Physical quantities can be extensive (depend on amount, e.g., mass, volume) or intensive (independent of amount, e.g., density, temperature).
Uncertainty in Measurement
Every measurement has an error or uncertainty due to limitations in instruments or observation.
Types of Errors:
- Systematic Errors: Consistent errors due to faulty equipment or procedure.
- Random Errors: Arising from unpredictable variations in measurement.
Precision refers to reproducibility of results, while accuracy means closeness to the true value.
Significant Figures: Reflect the precision of a measurement. For example, 2.54 cm has three significant figures.
Scientific Notation: Used to express large or small numbers conveniently, e.g., 6.022 × 1023.
Laws of Chemical Combinations
Chemistry follows several fundamental laws that govern chemical reactions:
- Law of Conservation of Mass: Mass can neither be created nor destroyed.
- Law of Definite Proportions: A compound always contains elements in a fixed ratio by mass.
- Law of Multiple Proportions: When two elements combine in different ratios, the masses of one element combine with a fixed mass of another in simple ratios.
- Gay-Lussac’s Law of Gaseous Volumes: Gases react in simple whole number ratios by volume under constant temperature and pressure.
- Avogadro’s Law: Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules.
These laws paved the way for atomic theory and quantitative chemistry.
Dalton’s Atomic Theory
Proposed by John Dalton in 1808, the atomic theory explains chemical combination based on the concept of atoms.
Postulates:
- All matter is made up of indivisible atoms.
- Atoms of a given element are identical in mass and properties.
- Atoms combine in simple whole-number ratios to form compounds.
- Chemical reactions involve rearrangement of atoms.
Though later modified (e.g., discovery of subatomic particles), Dalton’s theory remains foundational for modern atomic structure.
Atomic and Molecular Masses
Atomic Mass: The mass of an atom compared to 1/12th the mass of a carbon-12 atom. It is expressed in atomic mass units (u).
Molecular Mass: The sum of atomic masses of all atoms in a molecule. For example:
Molecular mass of H₂O = (2 × 1.008) + 16.00 = 18.016 u.
Formula Unit Mass: The mass of an ionic compound’s formula unit (e.g., NaCl = 58.5 u).
Mole Concept and Molar Masses
One mole represents 6.022 × 1023 (Avogadro number) entities — atoms, molecules, or ions.
Molar Mass: The mass of one mole of a substance, expressed in grams per mole (g/mol).
Relationships:
- Number of moles = Given mass / Molar mass
- Number of particles = Moles × 6.022 × 1023
This concept connects mass, number of particles, and volume of gases.
Percentage Composition
Percentage composition shows the proportion of each element in a compound by mass.
Formula:
% of element = (Mass of element in 1 mole of compound / Molar mass of compound) × 100
Example:
For H₂O: %H = (2 × 1) / 18 × 100 = 11.1%; %O = (16 / 18) × 100 = 88.9%.
It helps in determining empirical and molecular formulas.
Stoichiometry and Stoichiometric Calculations
Stoichiometry involves quantitative relationships between reactants and products in a chemical reaction.
Steps in Stoichiometric Calculations:
- Write a balanced chemical equation.
- Convert given quantities into moles.
- Use mole ratio from the equation.
- Calculate the required mass, volume, or number of particles.
Example:
2H₂ + O₂ → 2H₂O
If 4 g of H₂ reacts with excess O₂, moles of H₂ = 4/2 = 2 mol → produce 2 mol H₂O (36 g).
Stoichiometry forms the backbone of quantitative chemistry used extensively in JEE and NEET numerical problems.
Summary
Unit 1 introduces the foundational principles of chemistry that explain matter’s nature, its measurable properties, and quantitative relationships. Understanding these basics builds the framework for advanced topics like atomic structure, thermodynamics, and chemical bonding. Mastery of concepts like the mole, stoichiometry, and laws of combination is vital for competitive exams and real-world applications.
FAQs
Q1. Why is chemistry called the central science?**
Because it connects physical sciences like physics with life sciences such as biology, explaining how matter interacts and transforms.
Q2. What is the difference between physical and chemical properties?**
Physical properties can be observed without changing the substance (e.g., melting point), while chemical properties involve transformation (e.g., combustion).
Q3. What is the significance of the mole concept?**
It provides a link between the number of particles and measurable quantities like mass and volume, making chemical calculations easier.
Q4. Why are laws of chemical combinations important?**
They form the quantitative foundation of chemical reactions, explaining how elements combine in definite ratios to form compounds.
Q5. How do stoichiometric calculations help in real life?**
They are essential in industries for calculating yields, optimizing reactant use, and minimizing waste in chemical manufacturing.
Conclusion
Unit 1 establishes the base for understanding the entire subject of chemistry. It introduces matter, measurement, chemical laws, and quantitative relationships — the core tools of any chemist or engineer. Mastery of these fundamentals ensures clarity in later topics such as atomic structure, thermodynamics, and chemical equilibrium, which are crucial for exams like JEE and NEET as well as real-world scientific problem-solving.






Get Social