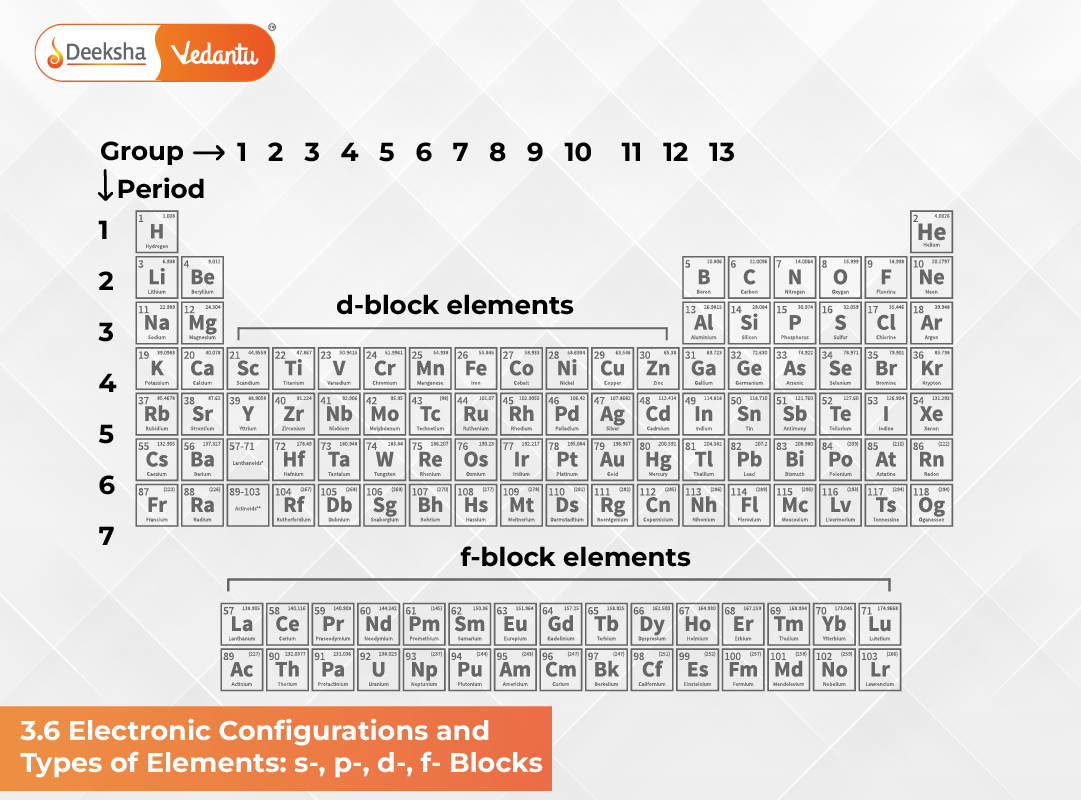
The electronic configuration of an atom provides the foundational logic behind the modern Periodic Table. According to the aufbau (build-up) principle, electrons fill atomic orbitals in increasing order of energy. Because elements with similar electron configurations exhibit similar chemical behaviours, the arrangement of electrons—especially in the outermost shells—allows us to group elements into meaningful categories.
The similarities in the electronic structures of atoms explain why certain sets of elements form natural families or groups. These recurring patterns provide the theoretical basis for classifying all elements into four major blocks: the s-block, p-block, d-block, and f-block, depending on the type of atomic orbital receiving the last electron. This block-wise classification not only simplifies the structure of the Periodic Table but also helps in predicting chemical properties, bonding patterns, and reactivity trends.
There are two important exceptions to this block classification:
- Helium, though having an ns² configuration, is placed in the p-block because of its fully filled valence shell and its chemical resemblance to noble gases.
- Hydrogen, with a single electron, does not fit perfectly into any one block. It can behave like an alkali metal (by losing an electron) or like a halogen (by gaining an electron). Therefore, it is placed separately at the top of the Periodic Table.
Below, we examine each block in detail.
3.6.1 The s-Block Elements
The elements in Groups 1 and 2 are collectively known as s-block elements because their valence electron(s) occupy the ns orbital. Their outermost configurations end in either ns¹ (alkali metals) or ns² (alkaline earth metals).
Characteristics of s-Block Elements
- They are highly reactive metals with low ionisation enthalpy.
- Alkali metals readily lose one electron to form M⁺ ions; alkaline earth metals form M²⁺ ions.
- They are soft, electropositive, and good reducing agents.
- Their metallic character and reactivity increase down the group.
- Most of their compounds (such as oxides, hydroxides, and halides) are predominantly ionic.
Because s-block elements lose electrons easily, they are rarely found in their free elemental state and must be stored carefully to prevent rapid reactions with air and moisture.
3.6.2 The p-Block Elements
The p-block comprises elements belonging to Groups 13–18. Their general valence shell configuration is ns²np¹–ns²np⁶. Along with the s-block, the p-block forms the representative or main group elements.
Key Features of p-Block Elements
- The number of valence electrons ranges from 3 to 8.
- They include metals, non-metals, and metalloids—the widest variety of properties in the Periodic Table.
- Many p-block non-metals (oxygen, nitrogen, halogens) form the basis of life-supporting biochemical cycles.
- The noble gases (Group 18) have completely filled valence shells, making them exceptionally stable and inert.
- The reactivity of p-block elements depends largely on whether they tend to gain, lose, or share electrons.
Trends such as electronegativity, metallic character, atomic size, and ionisation energy vary significantly across the p-block, making this region the most diverse section of the table.
3.6.3 The d-Block Elements (Transition Elements)
The d-block elements comprise Groups 3 to 12, where the (n–1)d orbitals gradually fill with electrons. These elements are commonly known as transition metals, reflecting the transitional behaviour between highly reactive s-block metals and the more variable p-block elements.
Properties of d-Block Elements
- They commonly exhibit variable oxidation states.
- Many form coloured ions due to d–d electronic transitions.
- They are excellent catalysts (e.g., Fe, Pt, Ni) because of their ability to form intermediate complexes.
- They have high melting and boiling points and are typically hard and dense.
- Their compounds often show paramagnetic behaviour due to unpaired d electrons.
The d-block introduces unique chemistry that cannot be observed in s- or p-block elements, making these metals essential in industrial, biological, and environmental applications.
3.6.4 The f-Block Elements (Inner Transition Elements)
The two rows at the bottom of the Periodic Table—the lanthanoids (Z = 57–71) and actinoids (Z = 89–103)—make up the f-block. These elements are characterised by the gradual filling of the (n–2)f orbitals.
Lanthanoids
- Known for forming stable +3 oxidation states.
- Exhibit sharp spectral lines used in lamps, lasers, and magnets.
- Have very similar chemical properties due to the “lanthanoid contraction.”
Actinoids
- Many elements are radioactive.
- They show a broader range of oxidation states compared to lanthanoids.
- The early actinoids display metallic and reactive behaviour; later ones are more stable.
Together, the lanthanoids and actinoids are referred to as inner transition elements. They are placed separately to keep the Periodic Table compact and readable.
3.6.5 Metals, Non-Metals, and Metalloids
Beyond the block classification, elements can also be grouped into metals, non-metals, and metalloids.
Metals
- Represent about 78% of all known elements.
- Are generally good conductors of heat and electricity.
- Are malleable and ductile.
- Have high melting and boiling points (with notable exceptions like mercury).
- Are positioned on the left and centre of the Periodic Table.
Non-Metals
- Typically poor conductors of heat and electricity.
- Mostly gases or brittle solids with low melting points.
- Tend to gain or share electrons during chemical reactions.
- Occupy the upper right corner of the Periodic Table.
Metalloids
- Have intermediate properties between metals and non-metals.
- Include elements such as silicon, germanium, and arsenic.
- Show a zig-zag arrangement separating metals and non-metals.
FAQs
Q1: What determines the block of an element?
The block of an element is determined by the type of orbital into which the final electron enters—s, p, d, or f.
Q2: Why is helium placed in the p-block despite its s² configuration?
Helium behaves chemically like a noble gas due to its completely filled valence shell, so it is placed in Group 18.
Q3: Why are lanthanoids and actinoids shown separately at the bottom of the Periodic Table?
They are placed separately to maintain a compact structure and because they share closely similar chemical properties.
Q4: What makes transition metals unique?
Their partly filled d-orbitals cause them to exhibit coloured ions, variable oxidation states, catalytic properties, and magnetic behaviour.
Q5: How are metalloids identified in the Periodic Table?
Metalloids lie along the zig-zag diagonal line separating metals from non-metals, showing mixed properties of both categories.
Conclusion
The classification of elements into s-, p-, d-, and f-blocks serves as a structured way to understand the underlying order of the Periodic Table. This block-wise grouping reflects how electrons fill different orbitals, leading to recurring patterns in chemical and physical properties. By exploring these categories, learners gain deeper insight into why elements behave the way they do and how electronic configurations shape the periodic trends we observe across the entire table.






Get Social