Organic compounds obtained from chemical reactions, natural sources or industrial processes are rarely pure. They often contain unreacted starting materials, by-products, catalysts, solvents or physical contaminants such as dust. These impurities can significantly change the melting point, boiling point, colour, odour, reactivity and stability of a substance. To study an organic compound’s true properties, perform further reactions, or use it in medicines and materials, we must first purify it.
From an exam point of view, purification is equally important. Many NCERT and competitive exam questions test whether you can identify the correct purification method for a given compound based on its physical and chemical properties. At Deeksha Vedantu, we train students to think in terms of: What is the state of the substance? Is it volatile, heat-sensitive, water soluble, organic soluble, or present in a mixture? Once these questions are answered, choosing the purification method becomes straightforward.
Why Purification Is Necessary
Purification plays a crucial role in:
- Confirming identity: Pure compounds have sharp melting and boiling points.
- Ensuring reliability: Impurities can interfere with reactions and give misleading results.
- Accurate characterisation: Techniques like spectroscopy and chromatography need pure samples.
- Scoring better in exams: Many theory and practical questions revolve around purification choices.
Common sources of impurities are:
- Incomplete reactions leaving reactants behind
- Side reactions forming by-products
- Oxidation or decomposition during heating
- Remaining solvents or drying agents
- Mechanical impurities such as dust or filter paper fibres
A strong conceptual grip on purification methods helps students confidently answer reasoning questions like “Why is this method chosen?” or “Which technique will give the best separation?”
Factors for Choosing a Purification Method
Before deciding on a method, we consider:
- Physical state: solid or liquid
- Volatility: does it sublime or distill easily?
- Thermal stability: does it decompose on heating?
- Solubility: in water, organic solvents, or both
- Amount present: bulk purification or small analytical sample
- Nature of impurity: volatile/non-volatile, soluble/insoluble, coloured/colourless
With these factors in mind, we now look at the major purification methods described in NCERT.
1. Sublimation
Sublimation is a convenient purification method for certain volatile solids which pass directly from the solid state to vapor on heating, without forming a liquid. Non-volatile impurities remain behind in the residue.
Principle
A solid that has sufficient vapour pressure at a temperature lower than its decomposition point will sublimate. When the vapour is cooled on a cold surface, it deposits back as a pure solid.
Suitable Compounds
Sublimation is suitable for:
- Naphthalene
- Camphor
- Anthracene
- Iodine
- Benzoic acid (in some cases)
- Solid ammonium chloride
Laboratory Setup and Steps
- Place the impure solid in a china dish.
- Cover the dish with an inverted funnel and plug the stem with cotton to prevent vapour escape.
- Heat the dish gently on a sand bath.
- The solid sublimes, and vapours rise inside the funnel.
- Vapours condense on the cooler walls of the funnel.
- After cooling, gently scrape off the purified crystals.
Example
A sample of naphthalene is contaminated with sand. Which technique would you use to purify it?
Since naphthalene sublimes on heating and sand is non-volatile, sublimation will separate naphthalene from sand.
Common Exam Pointers
- Only solids that sublime can be purified by this method.
- Non-sublimable impurities remain in the residue.
- Frequently used in one-mark reasoning questions.
Crystallisation
Crystallisation is the most widely used method to purify solid organic compounds. It is based on the difference in solubility of a substance in a solvent at high and low temperatures.
Principle
A suitable solvent should:
- Dissolve the impure solid at high temperature
- Dissolve very little of the solid at low temperature
- Either not dissolve impurities at all, or keep them completely in solution
When a hot, concentrated solution of the impure compound is cooled, the pure compound separates out as crystals while impurities remain in the filtrate or get removed in earlier filtration steps.
Steps in Crystallisation
- Selection of solvent: Choose a solvent that meets the solubility conditions and does not chemically react with the compound.
- Dissolving the solid: Heat the solvent and add the impure solid until it dissolves completely.
- Hot filtration: Filter the hot solution to remove insoluble impurities like dust.
- Cooling: Cool the clear hot solution slowly; crystals of the pure compound begin to form.
- Filtration of crystals: Filter off the crystals using a Buchner funnel or filter paper.
- Drying: Dry the crystals between filter papers or in a desiccator.
Choosing the Right Solvent – Key Conditions
- The compound must be considerably more soluble in hot solvent than in cold.
- Impurities should remain either insoluble (so they can be filtered off hot) or fully soluble (so they stay in the mother liquor).
- The solvent should evaporate easily and be inexpensive and non-toxic in a teaching laboratory.
Example
Benzoic acid is purified using hot water as the solvent. Explain why water is suitable for crystallising benzoic acid.
Benzoic acid is almost insoluble in cold water but appreciably soluble in hot water. Thus, on cooling its hot saturated solution, benzoic acid separates out as crystals, leaving soluble impurities behind.
Common Errors and Exam Tips
- Rapid cooling leads to small crystals that may trap impurities.
- Slow cooling gives larger, purer crystals.
- Crystallisation is often combined with charcoal treatment to remove coloured impurities.
Distillation
Distillation is used to purify liquid organic compounds or separate liquid mixtures. The method is based on boiling point differences.
A. Simple Distillation
Simple distillation is used when:
- A liquid contains non-volatile impurities, or
- Two miscible liquids have boiling points differing by more than about 25 °C.
In this method, the liquid is boiled, and the vapour is condensed in a condenser and collected in a receiver. The first liquid to distill is the one with the lower boiling point.
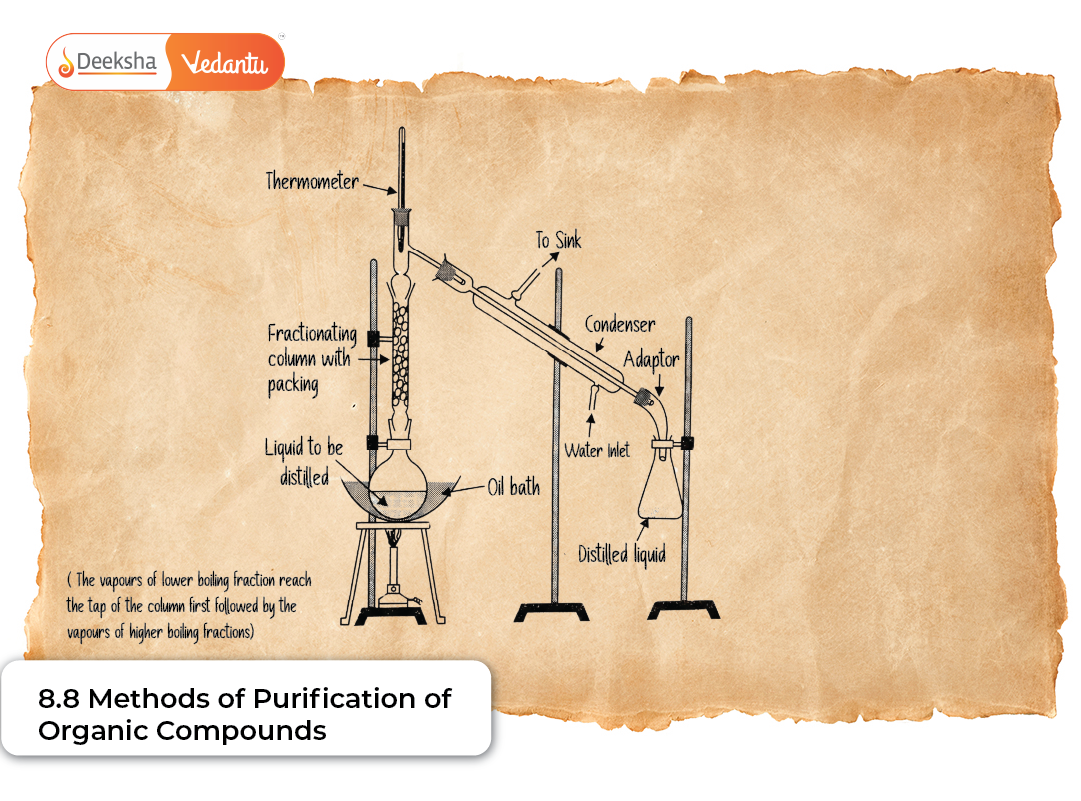
B. Fractional Distillation
Fractional distillation is used when components have closely spaced boiling points. A fractionating column filled with glass beads or plates is fitted between the distillation flask and condenser.
As vapours rise through the column, repeated condensation and vapourisation enrich the vapour in the more volatile component at each stage, leading to better separation.
Common Uses:
- Separation of ethanol and water
- Fractionation of petroleum into petrol, kerosene, diesel, etc.
C. Distillation Under Reduced Pressure (Vacuum Distillation)
Some organic liquids decompose at or near their normal boiling points. To distill them without decomposition, we reduce external pressure using a vacuum pump. This lowers their boiling points and allows safe distillation.
Example: Glycerol and high-boiling esters are often purified by vacuum distillation.
D. Steam Distillation
Steam distillation is applied to compounds that are:
- Immiscible with water
- Volatile in steam
- Likely to decompose at their boiling point if heated alone
In this technique, steam is passed through the mixture. The compound co-distils with water at a temperature below its normal boiling point. The distillate usually consists of two layers, which can then be separated.
Examples: Essential oils such as clove oil, aniline, and nitrobenzene.
Example
Why is steam distillation used to purify aniline?
Aniline is steam-volatile and tends to decompose near its boiling point. Steam distillation allows it to distill at a lower temperature along with water vapour, preventing decomposition.
Competitive Exam Pointer
Be very clear on which type of distillation to choose for a given situation-this is a favourite concept in assertion–reason and multiple-correct type questions.
Differential Extraction
Differential extraction (also called solvent extraction) separates compounds based on their different solubilities in two immiscible liquids, usually water and an organic solvent.
Principle
When an organic mixture is shaken with a suitable organic solvent, the component that is more soluble in the organic solvent will preferentially move into that layer. By carefully choosing solvents and using a separating funnel, we can move the desired substance from one phase to another.
Steps
- Place the aqueous solution or reaction mixture in a separating funnel.
- Add a measured amount of organic solvent (e.g., ether, chloroform).
- Stopper the funnel and shake gently, releasing pressure intermittently.
- Allow the layers to separate and identify the upper and lower layers based on density.
- Drain each layer separately.
- Evaporate the solvent from the organic layer to recover the purified compound.
Example
A mixture of benzoic acid and sodium chloride is present in water. How will you separate benzoic acid?
Benzoic acid is more soluble in organic solvents such as ether, while sodium chloride remains in the aqueous layer. Shaking the aqueous mixture with ether in a separating funnel will transfer benzoic acid into the ether layer, which can then be separated and evaporated to obtain purified benzoic acid.
Exam Tips
- Remember that ethers are less dense than water and form the upper layer.
- Halogenated solvents like chloroform and carbon tetrachloride are denser than water and form the lower layer.
Chromatography
Chromatography is a powerful technique for separating and purifying mixtures of substances present in small amounts. It exploits the different degrees to which components of a mixture are adsorbed on a stationary phase or partitioned between stationary and mobile phases.
Principle
When a mixture dissolved in a mobile phase (liquid or gas) is passed over a stationary phase (solid or liquid supported on a solid), the components move at different speeds due to differences in their affinity for the stationary phase. Components with stronger attraction to the stationary phase move more slowly and lag behind.
Types of Chromatography
A. Adsorption Chromatography
Here the stationary phase is a solid adsorbent (e.g., silica gel, alumina). The mixture is placed on the adsorbent and eluted with a solvent. Different components are adsorbed to different extents and thus separate into bands.
Column Chromatography: The adsorbent is packed in a column; the mixture is loaded at the top and eluted with a solvent. Components come out at different times as separate fractions.
B. Thin Layer Chromatography (TLC)
In TLC, a thin layer of adsorbent is coated on a glass or plastic plate. The mixture is spotted near the bottom, and the plate is placed in a solvent chamber. As the solvent rises, it carries the components to different heights.
The Rf value (retention factor) is used to identify components:
Rf = distance travelled by substance / distance travelled by solvent front
C. Partition / Paper Chromatography
In paper chromatography, water trapped in the pores of filter paper acts as the stationary phase, while an organic solvent serves as the mobile phase. Components distribute between the two phases based on partition coefficients and move at different rates.
Example
Why is chromatography considered a sensitive method of separation?
Chromatography can separate even very small quantities of closely related compounds due to subtle differences in their affinity for the stationary and mobile phases, making it highly sensitive and precise.
Common Uses
- Checking purity of compounds
- Separating plant pigments
- Monitoring reaction progress
Summary Table
| Method | Best For | Key Principle |
| Sublimation | Volatile solids | Solid → vapour without liquid phase |
| Crystallisation | Solid organic compounds | Solubility difference with temperature |
| Simple distillation | Liquids with large BP difference | Boiling point difference |
| Fractional distillation | Liquids with close BPs | Repeated condensation and vapourisation |
| Vacuum distillation | High-BP, heat-sensitive liquids | Lower BP under reduced pressure |
| Steam distillation | Steam-volatile organics | Co-distillation with steam |
| Differential extraction | Solutes in immiscible liquids | Different solubilities in two phases |
| Chromatography | Small-scale complex mixtures | Different affinities for stationary phase |
Practice Questions (Exam-Oriented)
- Which method would you use to purify a sample of camphor containing traces of non-volatile dust?
- a) Crystallisation
- b) Sublimation
- c) Simple distillation
- d) Steam distillation
Answer: b) Sublimation
- A mixture of two miscible liquids has a small difference in boiling points. Which method is most suitable for separation?
- a) Simple distillation
- b) Fractional distillation
- c) Sublimation
- d) Differential extraction
Answer: b) Fractional distillation
- Which technique would you use to check the purity of an organic compound quickly in the lab?
- a) Crystallisation
- b) Steam distillation
- c) Thin layer chromatography
- d) Vacuum distillation
Answer: c) Thin layer chromatography
- A solid organic acid is dissolved in water along with sodium chloride. How will you isolate the organic acid?
- a) Sublimation
- b) Differential extraction with ether
- c) Simple distillation
- d) Steam distillation
Answer: b) Differential extraction with ether
- Which of the following will be best purified by steam distillation?
- a) Sodium chloride
- b) Benzoic acid
- c) Aniline
- d) Glucose
Answer: c) Aniline
FAQs
Q1. How do I quickly decide which purification method to use in an exam?
Think about three properties: state (solid or liquid), volatility, and solubility. Volatile solids suggest sublimation, solids with good hot–cold solubility difference suggest crystallisation, liquids with different boiling points suggest distillation, water–organic systems suggest extraction, and tiny complex mixtures indicate chromatography.
Q2. Why is crystallisation preferred over sublimation for many solids?
Crystallisation can be applied to a wider range of solids, including non-volatile ones, and often gives very high purity. Sublimation is limited to solids that sublime readily and may be less suitable for non-sublimable substances.
Q3. How does steam distillation prevent decomposition of organic compounds?
In steam distillation, the organic compound co-distils with water at a temperature lower than its normal boiling point. Because it does not need to reach its full boiling point, the risk of decomposition is reduced.
Q4. What kind of questions on chromatography are common in competitive exams?
Exams often ask about Rf values, mobile and stationary phases, choice of solvent systems, and identification of components based on distance travelled on TLC or paper chromatograms.
Q5. Can a single method always give complete purification?
Not always. In practice, chemists often use a combination of methods-for example, crystallisation followed by chromatography-to achieve very high purity. In exams, however, you are generally expected to identify the most appropriate primary method.
Conclusion
Methods of purification form a core part of organic chemistry in Class 11 and beyond. Understanding sublimation, crystallisation, distillation, differential extraction and chromatography not only helps in performing experiments correctly but also builds strong problem-solving skills for board and competitive exams.
At Deeksha Vedantu, we connect each method with real-life laboratory practice, questions and exam patterns. This approach helps students choose purification methods logically, justify their answers clearly, and gain confidence in both theory and practical components of organic chemistry.






Get Social