Organic reaction mechanisms form the backbone of understanding how organic compounds transform during chemical reactions. They explain the sequence of steps, the movement of electrons, the breaking and formation of bonds, and the generation of short‑lived intermediates. This makes reaction mechanisms essential for predicting products, understanding reactivity, and performing higher-level organic reasoning.
NCERT Chapter 8.7 lays the foundation for all higher concepts of organic chemistry. At Deeksha Vedantu, we strengthen this foundation by using colourful arrow‑pushing animations, molecule rotation tools, stability comparison charts, and interactive reasoning exercises that help students not only understand but also visualise each reaction step clearly.
What Is a Reaction Mechanism?
A reaction mechanism is a stepwise account of how reactants convert into products. While a chemical equation only shows the final transformation, the mechanism answers deeper questions:
- Which bonds break first and why?
- Which bonds form next?
- How do electrons move between atoms?
- Why does a particular product dominate?
- Which intermediates appear and how stable are they?
Mechanisms convert organic chemistry from memorisation to logical problem solving.
Fission of Covalent Bonds
The breaking of covalent bonds is the first step in many reaction pathways. NCERT explains that covalent bonds break in two major ways – homolytic and heterolytic cleavage – each producing different species and leading to different types of reactions.
Homolytic Fission (Homolysis)
Homolysis occurs when the shared pair of electrons splits equally, giving one electron to each bonded atom.
Diagram: A-B → A· + B· (The dot on each species indicates one unpaired electron.)
Conditions promoting homolysis:
- UV radiation
- High heat
- Radical initiators such as peroxides
Why radicals are reactive
Free radicals contain unpaired electrons, making them eager to pair up during reactions. This eagerness drives chain reactions such as halogenation.
Examples of Homolytic Fission
- Chlorine dissociation under UV light: Cl₂ → 2Cl·
- Initiation step in methane chlorination: CH₄ + Cl₂ → CH₃· + HCl
These radicals move through propagation and termination steps, generating a chain mechanism.
Heterolytic Fission (Heterolysis)
In heterolysis, the shared electron pair moves entirely toward the more electronegative atom.
A-B → A⁺ + B⁻
This produces ions that participate primarily in polar reactions.
Conditions favouring heterolysis:
- Polar solvents (like water, alcohols)
- Strong polar bonds
- Presence of good leaving groups
Examples:
- Hydrogen halide ionisation: H-Cl → H⁺ + Cl⁻
- Alkyl halide cleavage: R-Br → R⁺ + Br⁻
The carbocation (R⁺) then undergoes nucleophilic attack.
Substrates and Reagents
Organic reactions involve a substrate reacting with one or more reagents.
Substrate
The molecule that undergoes chemical change.
Example: In SN2 reactions, CH₃–CH₂–Br acts as the substrate.
Reagent
The chemical species used to bring about the reaction.
Reagents may act as:
- Electrophiles
- Nucleophiles
- Oxidising agents
- Reducing agents
- Acids or bases
Types of Reagents and Their Functions
| Reagent Type | Examples | Function |
| Nucleophiles | OH⁻, RO⁻, NH₃, CN⁻ | Donate electron pairs |
| Electrophiles | H⁺, NO₂⁺, R⁺ | Accept electron pairs |
| Oxidising agents | KMnO₄, CrO₃ | Remove electrons |
| Reducing agents | LiAlH₄, NaBH₄ | Add electrons or hydride |
| Proton donors | H₂SO₄, HCl | Increase acidity |
Understanding these roles helps classify and predict reaction behaviour.
Electron Movement (Curved Arrow Notation)
Mechanisms rely on arrows to show how electrons move.
Types of Curved Arrows
Full-headed arrow
Shows movement of an electron pair.
Half-headed arrow
Shows movement of a single electron (radical pathways).
Why arrow notation matters
- Clearly shows electron donors and acceptors.
- Helps identify which steps form or break bonds.
- Shows direction of nucleophilic attack.
- Predicts possible side reactions.
Nucleophiles and Electrophiles
Reactivity in organic chemistry revolves around interactions between electron‑rich nucleophiles and electron‑deficient electrophiles.
Nucleophiles
Nucleophiles donate electrons and attack electron-deficient centres.
Characteristics
- Often negatively charged
- Contain lone pairs or π electrons
- Stronger nucleophiles are usually more basic (exceptions exist)
Examples of Nucleophiles
- HS⁻
- I⁻
- Enolates
- Aromatic rings (when activated)
Electrophiles
Electrophiles accept electrons.
Characteristics
- Positively charged or partially positive
- Attracted to electron-rich regions
Examples of Electrophiles
- SO₃
- Br₂ (polarised)
- AlCl₃ (Lewis acid)
- Carbonyl carbon in aldehydes/ketones
Detailed Comparison
| Property | Nucleophiles | Electrophiles |
| Charge | Often negative | Often positive |
| Electrons | Rich | Deficient |
| Function | Attack electrophiles | Accept electrons |
| Common in | SN2, addition | SN1, E1, EAS |
| Examples | OH⁻, CN⁻, NH₃ | H⁺, NO₂⁺, R⁺ |
Reactive Intermediates
Reactive intermediates form transiently during reactions and govern the rate and course of mechanisms.
Free Radicals
Properties:
- Possess an unpaired electron
- Paramagnetic
- Show high reactivity
- Exhibit radical stability trends similar to carbocations
Stability Order
3° > 2° > 1° > CH₃·
Real-world example
Polymerisation of ethene involves radical chain processes.
Carbocations (Carbenium Ions)
Carbocations have:
- sp² hybridisation
- Planar structure
- An empty p-orbital for electron acceptance
Formation
- Via heterolytic cleavage
- Protonation of alkenes
- Solvolysis of alkyl halides
Expanded Stability Order
3° (most stable due to hyperconjugation) > 2° > 1° > CH₃⁺ (least stable)
Additional Stabilisation
- Resonance stabilisation (e.g., benzyl, allyl carbocations)
Carbanions
Carbanions contain a carbon with three bonds and one lone pair.
Properties
- Highly basic
- Strong nucleophiles
- Pyramidal geometry
Additional Stability Order
CH₃⁻ > 1° > 2° > 3° (due to electron repulsion by alkyl groups)
Carbenes and Nitrenes
Though introduced fully later in NCERT, they influence many advanced mechanisms.
Carbenes
- Neutral divalent carbon
- Singlet or triplet forms
Nitrenes
- Neutral nitrogen analogues of carbenes
Electronic Effects
Reactivity is dictated not just by structure but by how electrons shift within molecules.
Inductive Effect (I-Effect)
Electron displacement through σ bonds due to electronegativity.
Expanded Examples
- –NO₂ (strong –I)
- –F > –Cl > –Br (halogens differ in –I strength)
- Alkyl groups (+I)
Application
- Predicting acidity/basicity
- Stabilising/destabilising carbocations and carbanions
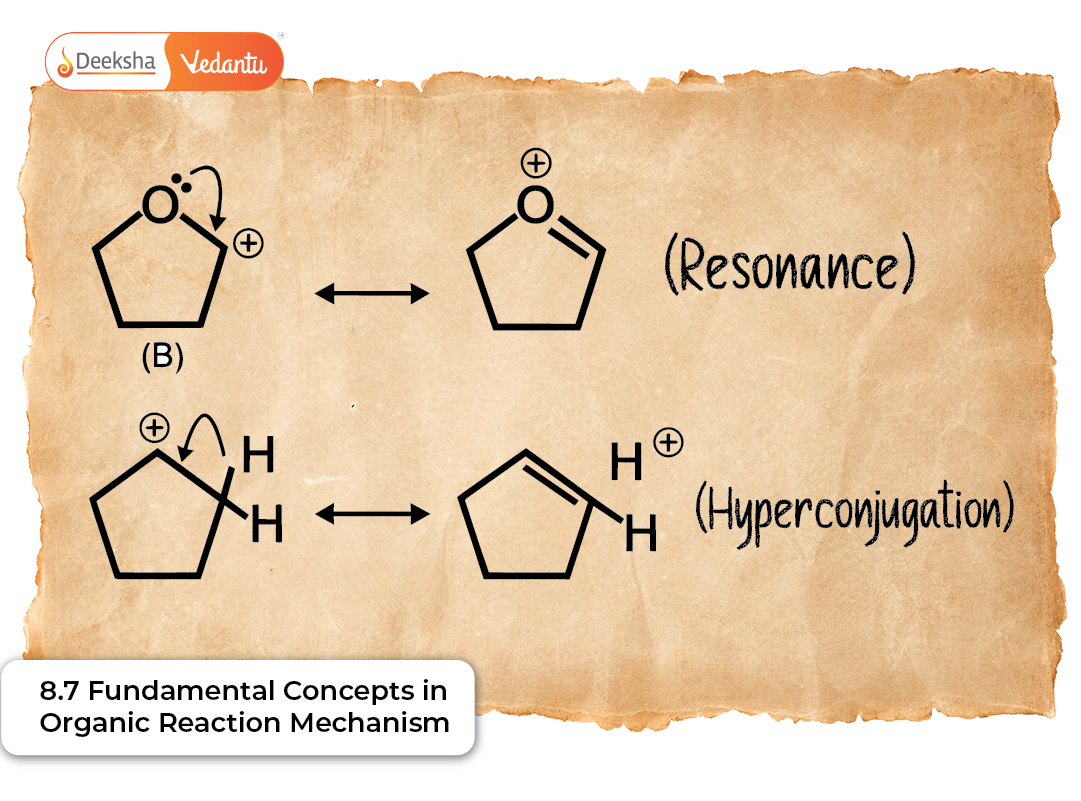
Resonance (Mesomeric Effect)
Delocalisation of π electrons across conjugated systems.
Detailed Examples
- Phenoxide ion resonance
- Nitro group resonance structures
- Allylic systems
Diagram-style Illustration
O⁻ ↔ O= (phenoxide ion)
Hyperconjugation
Also called “no-bond resonance,” involving σ (C–H) → empty p-orbital overlap.
Notes
- Explains stability of substituted alkenes
- Gives extra stability to carbocations
- Influences alkene geometries
Summary of Electronic Effects
| Effect | Nature | Examples | Influence |
| –I | Electron-withdrawing | NO₂, CN, halogens | Decreases electron density, stabilises carbocations, increases acidity |
| +I | Electron-donating | Alkyl groups | Increases electron density, stabilises carbanions, increases basicity |
| +R | Electron-releasing via resonance | –OH, –NH₂ | Stabilises carbocations and electron-deficient centres |
| –R | Electron-withdrawing via resonance | –NO₂, –CHO | Pulls electron density away, activates electrophilic positions |
Types of Organic Reactions
Organic reactions are broadly classified based on how bonds break and form. Below is a significantly expanded view aligned with NCERT and competitive exam expectations.
Substitution Reactions
A group in the substrate is replaced by another group.
Types:
- SN1 (unimolecular nucleophilic substitution) – proceeds via carbocation intermediate.
- SN2 (bimolecular nucleophilic substitution) – single-step backside attack.
Example of SN2:
OH⁻ + CH₃–Br → CH₃–OH + Br⁻
Example of SN1:
(t-Bu)–Br → (t-Bu)⁺ → nucleophile attack → substitution product.
Addition Reactions
Typically occur in unsaturated systems like alkenes and alkynes.
Examples:
- Addition of HBr to propene.
- Hydrogenation of alkenes using Ni catalyst.
- Hydration of ethene to form ethanol.
Elimination Reactions
Two atoms/groups are removed, usually forming a double bond.
Key Types:
- E1 mechanism – two-step via carbocation.
- E2 mechanism – one-step elimination.
Example:
CH₃–CH₂–Br + KOH (alc.) → CH₂=CH₂ + HBr
Rearrangement Reactions
Atoms within a molecule shift to form more stable intermediates.
Common rearrangements:
- Hydride shift
- Methyl shift
- Wagner–Meerwein rearrangement
Example:
Carbocation rearrangement of 2-methyl-1-butanol during dehydration.
Redox Reactions
Involve oxidation or reduction of carbon centres.
Examples:
- Oxidation of primary alcohols to aldehydes.
- Reduction of aldehydes using NaBH₄.
Solved Examples
These examples help reinforce concepts from NCERT while aligning with JEE/NEET reasoning.
Example 1: Predict Major Product Using Electronic Effects
Reaction: Propene + HBr → ?
Due to Markovnikov’s rule, H⁺ adds to the carbon with more H, forming the more stable carbocation intermediate. Product: 2-bromopropane.
Example 2: Identify Reaction Type
CH₃–CH₂–OH → CH₂=CH₂ (conc. H₂SO₄, heat)
This is an elimination (E1) reaction.
Example 3: Determine Nucleophile Strength
Rank the nucleophiles: OH⁻, H₂O, RO⁻
Strength: RO⁻ > OH⁻ > H₂O.
Example 4: Stability of Carbocations
Rank stability: Allyl⁺, tert-butyl⁺, benzyl⁺
Correct order: Benzyl⁺ ≈ Allyl⁺ (resonance-stabilised) > tert-butyl⁺.
FAQs
Q1. What is the main difference between homolytic and heterolytic cleavage?
Homolytic cleavage generates radicals; heterolytic cleavage produces ions.
Q2. Why are reactive intermediates important?
They determine reaction rate, stability, and pathways.
Q3. How do nucleophiles and electrophiles influence reactions?
Nucleophiles donate electrons while electrophiles accept them, driving bond formation.
Q4. Why is resonance significant?
It distributes electron density across multiple atoms, increasing stability.
Q5. Why is arrow-pushing notation crucial in mechanisms?
It visually reveals electron movement, making mechanisms easier to follow.
Conclusion
Mastering these fundamental concepts transforms organic chemistry into a logical and predictable subject. By analysing electron movement, understanding intermediates, and interpreting electronic effects, students can confidently predict reaction outcomes and excel in competitive exams. At Deeksha Vedantu, our structured visuals and guided learning approach make even complex mechanisms easy to understand.





Get Social