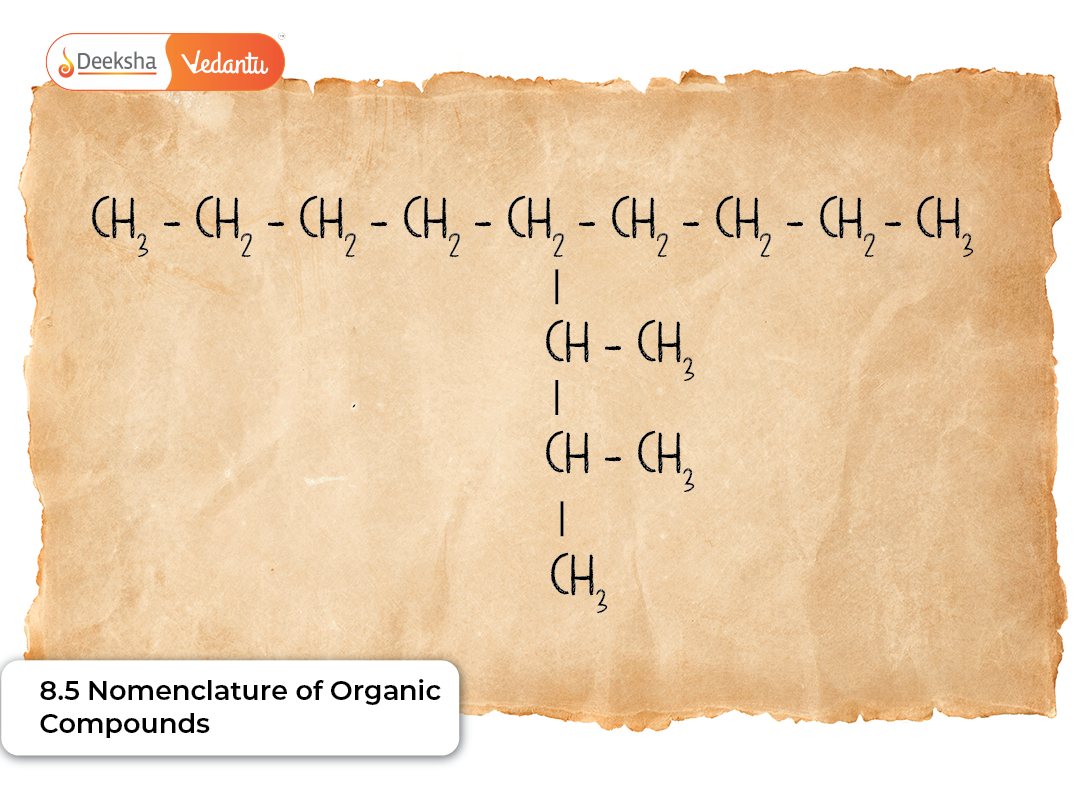
Organic compounds exist in extraordinarily large numbers, and without a clear, universally accepted naming system, it would be nearly impossible for chemists to communicate accurately. The nomenclature of organic compounds provides a systematic and standardised way of naming molecules so that each compound corresponds to only one correct name. NCERT Class 11 Chapter 8.5 introduces the foundations of the IUPAC system, enabling students to interpret and construct names based on structural features.
At Deeksha Vedantu, we break down the nomenclature process into simple rules, visual strategies, and practice-oriented learning. Students are trained to identify structures, assign correct names, avoid common mistakes, and understand the logic behind each naming decision-skills essential for board exams and competitive preparation.
Why Nomenclature Is Important
Organic chemistry involves millions of compounds, many of which differ only slightly in structure. A precise naming system helps:
- Eliminate confusion and ambiguity in chemical communication.
- Reveal key structural details directly through the name.
- Improve understanding of functional groups and their behaviour.
- Allow systematic learning of reaction mechanisms and properties.
- Provide a universal standard followed globally in research, academia, and industry.
The IUPAC system links names to structures logically, making it easier for students to decode or construct names even when encountering new molecules.
Basic Principles of IUPAC Nomenclature
Every IUPAC name contains three essential components:
- Prefix – indicates substituents or side chains attached to the main chain.
- Word root – indicates the number of carbons in the longest chain.
- Primary suffix – indicates saturation or unsaturation (–ane, –ene, –yne).
- Secondary suffix – indicates the principal functional group (–ol, –al, –one, –oic acid).
Some common word roots include:
- 1 carbon → meth
- 2 carbons → eth
- 3 carbons → prop
- 4 carbons → but
- 5 carbons → pent
- 6 carbons → hex
- 7 carbons → hept
A name like propan-2-ol tells us the molecule is a three‑carbon chain (prop), is saturated (an), has an –OH group (ol), and the functional group is located on carbon 2.
Step-by-Step Rules for Naming Organic Compounds
Step 1: Identify the Longest Carbon Chain
Choose the carbon chain that contains the maximum number of carbons. If two chains have equal length, the chain with more substituents or more significant functional groups is selected.
Example:
CH₃–CH₂–CH(CH₃)–CH₃
Longest chain = 4 carbons → butane
Step 2: Number the Carbon Chain
Numbering begins from the end closest to the functional group or first branching point.
Rules:
- Functional groups get the lowest possible number.
- Double/triple bonds get priority over substituents.
- If there is still a tie, the alphabetical order of substituents decides the direction.
Step 3: Identify and Name Substituents
Substituents are atoms or groups attached to the main chain.
Common substituents:
- –CH₃ → methyl
- –C₂H₅ → ethyl
- –Cl → chloro
- –Br → bromo
- –NO₂ → nitro
- –NH₂ → amino
When multiple identical substituents appear, prefixes such as di, tri, tetra are used.
Step 4: Assign Locants (Position Numbers)
Each substituent or multiple bond must have a locant showing its position.
Examples:
- 2-methylbutane
- 3,3-dimethylpentane
Step 5: Assemble the Name in Correct Order
The general format is: Locant + Prefix + Word Root + Primary Suffix + Secondary Suffix
Alphabetical order applies only to prefixes, not multiplicative terms.
IUPAC Naming Examples
Example 1: Branched Alkane
Structure: CH₃–CH(CH₃)–CH₂–CH₃
- Longest chain = 4 carbons → butane
- Substituent = CH₃ on carbon 2 → 2-methyl
IUPAC Name: 2-methylbutane
Example 2: Alkene
Structure: CH₃–CH=CH–CH₃
- Root = but
- Double bond starts at carbon 2 → 2-ene
IUPAC Name: but-2-ene
Example 3: Alkyne
Structure: CH≡C–CH₂–CH₃
- Root = but
- Triple bond at carbon 1 → 1-yne
IUPAC Name: but-1-yne
Example 4: Alcohol
Structure: CH₃–CH₂–CH₂–OH
- Root = prop
- –OH at carbon 1 → 1-ol
IUPAC Name: propan-1-ol
Example 5: Aldehyde
Structure: CH₃–CH₂–CHO
- Aldehyde group always gets position 1.
IUPAC Name: propanal
Example 6: Ketone
Structure: CH₃–CO–CH₃
- Root = prop
- Ketone at carbon 2 → 2-one
IUPAC Name: propan-2-one
Naming Aromatic Compounds
Aromatic compounds are based on the benzene ring. The simplest derivatives include:
- Methylbenzene (toluene)
- Chlorobenzene
- Nitrobenzene
When substituents are present, numbering follows the lowest locant rule.
Example:
Structure: 1,3-dichlorobenzene
Substituents at positions 1 and 3.
IUPAC Name: 1,3-dichlorobenzene
If only two substituents are present, traditional names such as ortho (o‑), meta (m‑), para (p‑) may be used, but IUPAC numeric naming is still preferred.
Naming Compounds with Multiple Functional Groups
Functional groups follow a priority order. The group with the highest priority becomes the suffix, while the others are written as prefixes.
Priority order (highest to lowest):
- Carboxylic acids
- Aldehydes
- Ketones
- Alcohols
- Amines
Example:
CH₃–CH(OH)–COOH
Primary FG: –COOH → oic acid
Secondary FG: –OH → hydroxy
IUPAC Name: 2-hydroxypropanoic acid
Another Example:
CH₃–CO–CH₂–NH₂
- Ketone is higher priority than amine
- Root = propan
- Functional group: one
- Prefix: amino
IUPAC Name: 3-aminopropan-2-one
Additional Naming Scenarios
Nomenclature of Substituted Alkanes
Substituted alkanes contain one or more side chains or groups attached to the main alkane chain. The naming focuses on identifying the parent chain, numbering for lowest locants, and listing substituents alphabetically.
Example:
Structure: CH₃–CH₂–CH(CH₃)–CH₂–CH₃
- Longest chain: pentane
- Substituent: methyl at C-3
IUPAC Name: 3-methylpentane
If multiple substituents are present, their locants are mentioned individually.
Example:
Structure: CH₃–CH(CH₃)–CH(CH₃)–CH₃
- Two methyl groups at C-2 and C-3
IUPAC Name: 2,3-dimethylbutane
Nomenclature of Alkenes and Alkynes
Double and triple bonds must be included in the parent chain. Numbering ensures the multiple bond receives the lowest possible number.
Example (Alkene):
CH₂=CH–CH₂–CH₃ → but-1-ene
Example (Alkyne):
CH≡C–CH₂–CH₃ → but-1-yne
If both double and triple bonds exist, the suffix becomes –en–yne.
Example:
CH₂=CH–C≡CH → but-1-en-3-yne
Nomenclature of Compounds with Functional Groups
Functional groups significantly influence naming because they act as the foundation for suffix selection.
Secondary suffixes:
- –ol (alcohol)
- –al (aldehyde)
- –one (ketone)
- –oic acid (carboxylic acid)
Example:
CH₃–CH₂–OH → ethanol CH₃–CHO → ethanal CH₃–CO–CH₃ → propan-2-one
If functional groups are used as prefixes:
- –OH → hydroxy
- –NH₂ → amino
- –CHO → formyl
Nomenclature of Halogen Derivatives
Halogen groups (F, Cl, Br, I) are treated as substituents.
Examples:
CH₃–CH₂–Cl → chloroethane CH₃–CHCl–CH₃ → 2-chloropropane
Multiple halogens are listed alphabetically.
Example:
CHBrCl–CH₃ → 1-bromo-1-chloroethane
Nomenclature of Substituted Benzene Compounds
When one substituent is attached, the compound is named as a monosubstituted benzene.
Examples:
- C₆H₅CH₃ → methylbenzene (toluene)
- C₆H₅Cl → chlorobenzene
When two substituents are present, positions are denoted using 1,2, 1,3, 1,4 or ortho, meta, para.
Examples:
- 1,2-dichlorobenzene (o-dichlorobenzene)
- 1,3-nitromethylbenzene (m-nitrotoluene)
Nomenclature of Polyfunctional Compounds
When multiple functional groups are present, NCERT lists a prioritised order. The highest priority group becomes the suffix.
Priority example:
COOH > SO₃H > COOR > COX > CONH₂ > CHO > CO > OH > NH₂ > NO₂
Example:
HOOC–CH₂–CH₂–OH
- Primary FG: –COOH → suffix
- –OH → hydroxy
Name: 3-hydroxypropanoic acid
NCERT Emphasis: Word Root, Primary Suffix, Secondary Suffix
NCERT highlights the importance of understanding suffixes:
- Primary suffix shows saturation (–ane), unsaturation (–ene, –yne)
- Secondary suffix represents the main functional group
- Prefixes represent substituents
Example:
CH₃–CH₂–COOH
- Root = prop
- Primary suffix = an
- Secondary suffix = oic acid
IUPAC Name: propanoic acid
This deeper understanding allows students to decode any structure into a correct IUPAC name.
Compounds with Multiple Double or Triple Bonds
Example:
CH₂=CH–C≡CH
- Double bond at carbon 1 → 1-ene
- Triple bond at carbon 3 → 3-yne
Name: but-1-en-3-yne
Cyclic Hydrocarbons
- Cyclohexane, cyclopentene, etc.
- Substituents are numbered to give the lowest possible numbers.
Example:
- CH₃ attached to cyclohexane.
- Name: methylcyclohexane
FAQs
Q1. Why do we use IUPAC names instead of common names?
IUPAC names follow a universal, logical system that removes ambiguity and helps identify structures.
Q2. What is the first step in naming an organic compound?
Identifying the longest continuous carbon chain.
Q3. How do we name multiple substituents?
Use alphabetical order and assign the lowest possible locant numbers.
Q4. What if two functional groups are present?
The higher-priority group becomes the suffix, while others become prefixes.
Q5. Do aromatic compounds follow the same rules?
Yes, but numbering starts from the substituent providing the lowest locants.
Conclusion
Nomenclature acts as the language of organic chemistry, turning complex structures into clear, meaningful names. Mastering IUPAC rules helps students interpret mechanisms, identify compounds, and approach organic chemistry with strong conceptual understanding.
At Deeksha Vedantu, we ensure that students learn nomenclature through structured steps, ample practice, and personalised explanation-building a solid base for future organic chemistry topics like isomerism, stereochemistry, and reaction mechanisms.





Get Social