The modern periodic table that we use today is the culmination of several discoveries spanning over a century. Early chemists arranged elements based on atomic mass because the internal structure of atoms was not yet understood. Although Mendeleev’s periodic table was revolutionary for its time, several inconsistencies appeared when new elements were discovered. A major leap occurred in the early 20th century when Henry Moseley proved that atomic number—not atomic mass—is the fundamental property governing the arrangement of elements. This discovery reshaped the entire periodic table, giving rise to what we now call the Modern Periodic Law.
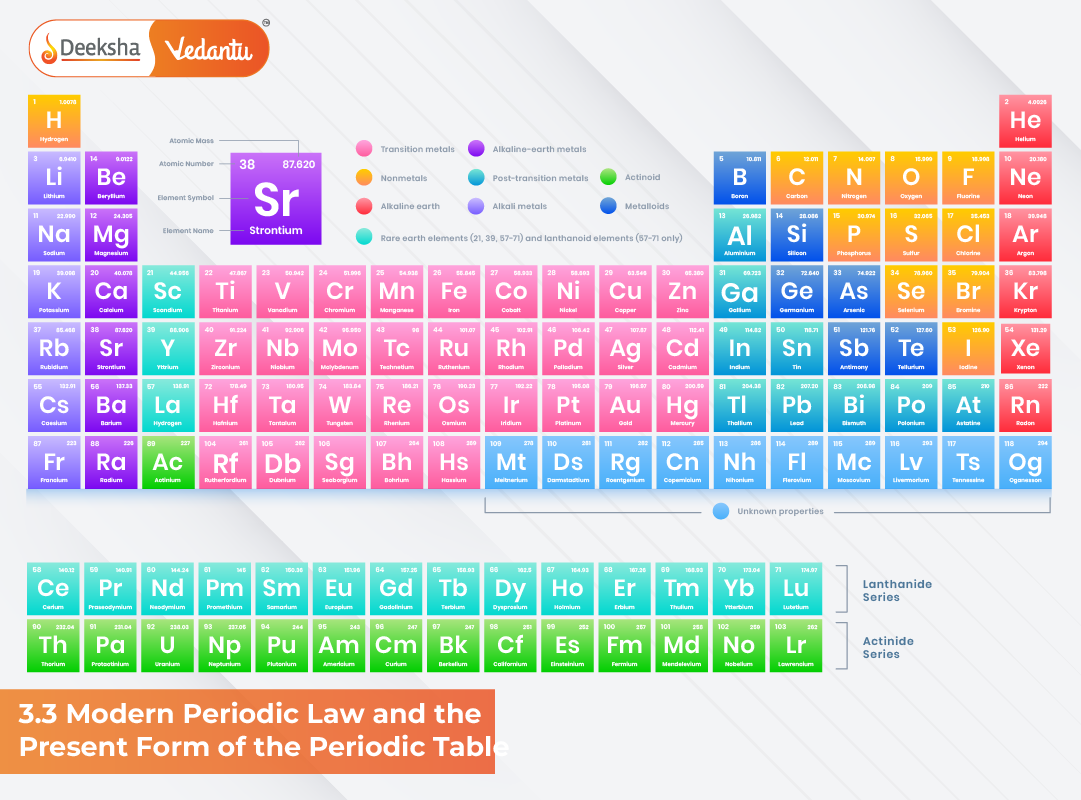
The present form of the periodic table is more systematic, logical, and scientifically supported. It reflects periodicity based on electron configurations and provides a clear framework for predicting the physical and chemical behaviour of elements. With seven periods, eighteen groups, and well-defined blocks (s, p, d, f), the table showcases recurring patterns that stem from the arrangement of electrons in atoms.
Modern Periodic Law
Modern Periodic Law states that the physical and chemical properties of elements are periodic functions of their atomic numbers. This means that when elements are arranged in order of increasing atomic number, their properties repeat at definite intervals. The atomic number, which represents the number of protons in the nucleus, uniquely defines each element and influences its electron configuration. As chemical properties depend largely on valence electrons, repeating patterns arise naturally when atomic numbers increase.
This law resolved conflicting placements of several elements in earlier tables. For example, argon (with a higher atomic mass) comes before potassium because its atomic number is lower. Isotopes, which have different masses but identical atomic numbers, also fit seamlessly into the same position—something that atomic mass-based classification could not explain.
Moseley’s Contribution
Before Moseley’s work, chemists relied heavily on atomic masses to arrange elements. In 1913, Henry Moseley used X-ray spectroscopy to study the frequencies emitted by various elements. He observed that these frequencies increased in a regular manner with rising nuclear charge. When plotted, the square root of frequency showed a linear relationship with atomic number. This was a clear indication that atomic mass was not the true basis of periodicity.
Through this discovery, Moseley established that atomic number is equal to the positive charge in the nucleus. This revolutionary idea corrected earlier misplacements such as cobalt and nickel and ensured that newly discovered elements could be placed accurately in the table. His findings also provided theoretical support for periodic trends like ionization enthalpy, electronegativity, and atomic radius, which depend on effective nuclear charge.
Structure of the Modern Periodic Table
The present-day periodic table is often referred to as the long-form periodic table because it spreads elements more widely across 18 vertical columns. This structure highlights the role of electron configurations and illustrates periodicity clearly. Each element is placed according to:
- its atomic number,
- its electronic configuration,
- recurring periodic trends.
The table’s layout helps us identify families of elements with shared properties, interpret trends across periods, and understand the effect of nuclear charge on atomic behaviour.
Periods
Periods are the horizontal rows in the periodic table. There are seven periods, each corresponding to the filling of a new principal energy shell. As one moves from left to right across a period, the nuclear charge increases while electrons are added to the same shell, resulting in gradual changes in properties.
Properties that show clear trends across periods include:
- Metallic to non-metallic transition: Elements start as metals on the left and gradually become non-metals toward the right.
- Decrease in atomic radius: Increasing nuclear charge pulls electrons closer.
- Increase in ionization enthalpy: More energy is required to remove an electron.
- Variation in electronegativity: Non-metals typically have higher electronegativity.
Each period marks the beginning of a new energy level, and the number of elements in a period increases as the capacity of subshells grows.
Groups
Groups are the vertical columns of the periodic table. The modern periodic table has 18 groups, and all elements within the same group share similar valence electron configurations. This similarity gives them nearly identical chemical properties.
Some major groups include:
- Group 1 – Alkali metals: Highly reactive, soft metals.
- Group 2 – Alkaline earth metals: Less reactive than Group 1 but still form strong bases.
- Group 17 – Halogens: Highly reactive non-metals forming salts with metals.
- Group 18 – Noble gases: Chemically inert due to stable electronic configurations.
Trends within groups include:
- Increasing atomic radius down the group due to added electron shells.
- Decreasing ionization enthalpy as outer electrons are farther from the nucleus.
- Increasing metallic character from top to bottom.
Periodicity and Its Basis
Periodic trends arise because elements with repeating valence electron configurations appear at regular intervals in the periodic table. These patterns form the basis of periodicity. Some of the most important periodic properties include:
- Atomic radius – generally decreases across a period and increases down a group.
- Ionic radius – depends on charge and number of electrons.
- Ionization enthalpy – increases across a period due to stronger attraction between nucleus and electrons.
- Electron gain enthalpy – becomes more negative across a period for non-metals.
- Electronegativity – highest for elements near the top right of the table.
Understanding these properties helps predict reactivity, bonding behavior, acidity/basicity, oxidation states, and general trends observed across the periodic table.
Long Form of the Periodic Table
The long-form periodic table is based on the order of orbital filling described by the Aufbau principle. It organizes elements into four blocks:
- s-block: Groups 1 and 2; elements with outer electrons in s-orbitals.
- p-block: Groups 13 to 18; elements with outer electrons in p-orbitals.
- d-block: Transition metals; elements with partially filled d-orbitals.
- f-block: Lanthanides and actinides; elements with electrons in f-orbitals.
This classification explains why elements in the same block often show similar characteristics. For example, d-block elements typically exhibit variable oxidation states and form colored compounds, whereas s-block elements show strong reducing behavior.
The long form also separates inner transition metals (f-block) to maintain the structure’s clarity while preserving numerical order.
FAQs
Q1. What is Modern Periodic Law?
Modern Periodic Law states that the physical and chemical properties of elements are periodic functions of their atomic numbers.
Q2. Who discovered the atomic number?
Henry Moseley discovered the atomic number through his work on X-ray spectra.
Q3. How many groups and periods are there in the periodic table?
There are 18 groups and 7 periods in the modern periodic table.
Q4. Why was Moseley’s discovery significant?
Moseley’s discovery corrected inconsistencies in Mendeleev’s table and provided a scientific basis for arranging elements by atomic number.
Q5. What are blocks in the periodic table?
Blocks divide elements into s, p, d, and f categories based on the type of subshell receiving the last electron.
Conclusion
The Modern Periodic Law and present-day periodic table provide a structured and accurate framework for classifying elements. By basing the arrangement on atomic number and electron configuration, the modern table resolves earlier inconsistencies and highlights clear periodic trends. Its organisation into periods, groups, and blocks makes it an invaluable tool in studying chemical behaviour, predicting reactions, and understanding the underlying patterns governing the properties of elements.





Get Social