Hybridization is a foundational concept in modern chemistry that explains how atoms form directional and stable bonds by combining atomic orbitals of nearly equal energies. It is a crucial part of Valence Bond Theory and helps in visualising the shapes and geometries of molecules. This concept becomes especially relevant while studying molecules like CH₄, NH₃, H₂O, C₂H₂ and BCl₃, where simple orbital overlap does not sufficiently explain the observed bond angles and geometries. According to Linus Pauling, hybridization is the process in which atomic orbitals mix and reorganise to produce a new set of hybrid orbitals that have equivalent energy and shape and are capable of forming stronger covalent bonds.
Hybridization not only explains the geometry of molecules but also helps in understanding bond strength, bond angles and electron density distribution around the atoms. For instance, when one s orbital and three p orbitals in carbon hybridise, they form four sp³ hybrid orbitals that arrange themselves in a tetrahedral geometry to minimise repulsion. This concept also supports the VSEPR theory, making it an essential tool for predicting molecular shapes in NCERT, NEET and JEE syllabi.
Salient Features of Hybridization
The process of hybridization exhibits certain important characteristics:
- The total number of hybrid orbitals formed is always equal to the number of atomic orbitals participating in hybridization.
- All hybrid orbitals formed have the same energy and shape, making them equivalent for bond formation.
- Hybrid orbitals provide greater overlap, resulting in stronger covalent bonds than pure atomic orbitals.
- The hybrid orbitals are oriented in specific directions in space to reduce electron pair repulsion, leading to stable molecular geometries.
- Promotion of electrons to higher energy levels may occur to facilitate hybridization, but it is not mandatory in all cases.
- Hybridization helps in understanding both central atom geometry and bond strength, especially in polyatomic molecules.
- Lone pairs occupying hybrid orbitals influence bond angles and molecular shape, as seen in NH₃ and H₂O.
Important Conditions for Hybridization
Hybridization will take place only when certain conditions are fulfilled:
- The orbitals must belong to the same valence shell of an atom.
- The atomic orbitals involved in hybridization should possess comparable energy levels.
- The orbitals that undergo hybridization should be capable of participating in effective overlap for bond formation.
- In some cases, even fully filled orbitals may undergo hybridization if it leads to increased molecular stability.
- The resulting hybrid orbitals must be oriented in space to ensure maximum overlap and minimum repulsion, creating stable molecular geometry.
These conditions collectively determine whether hybridization is possible and what geometry a molecule will adopt.
Types of Hybridization
Depending on the type and number of atomic orbitals involved, different types of hybridization are observed:
1. sp Hybridization (Linear Geometry)
In sp hybridization, one s orbital combines with one p orbital to form two sp hybrid orbitals that lie 180° apart. This leads to a linear geometry. It is seen in molecules like BeCl₂ and C₂H₂.
- Bond angle: 180°
- Shape: Linear
- Example molecules: BeCl₂, C₂H₂
2. sp² Hybridization (Trigonal Planar Geometry)
Here, one s orbital mixes with two p orbitals to form three sp² hybrid orbitals that lie in one plane with a 120° separation. This results in a trigonal planar geometry.
- Bond angle: 120°
- Shape: Trigonal planar
- Example molecule: BCl₃
3. sp³ Hybridization (Tetrahedral Geometry)
In this case, one s orbital and three p orbitals mix to form four sp³ hybrid orbitals arranged in three-dimensional space with a bond angle of 109.5°, forming a tetrahedral structure.
- Bond angle: 109.5°
- Shape: Tetrahedral
- Example molecules: CH₄, NH₃, H₂O (with distorted geometry due to lone pairs)
4. sp³d Hybridization (Trigonal Bipyramidal Geometry)
This type of hybridization involves one s orbital, three p orbitals and one d orbital to create five sp³d hybrid orbitals. They form a trigonal bipyramidal geometry, commonly seen in PCl₅.
- Bond angles: 120° (equatorial) and 90° (axial)
- Shape: Trigonal bipyramidal
- Example molecule: PCl₅
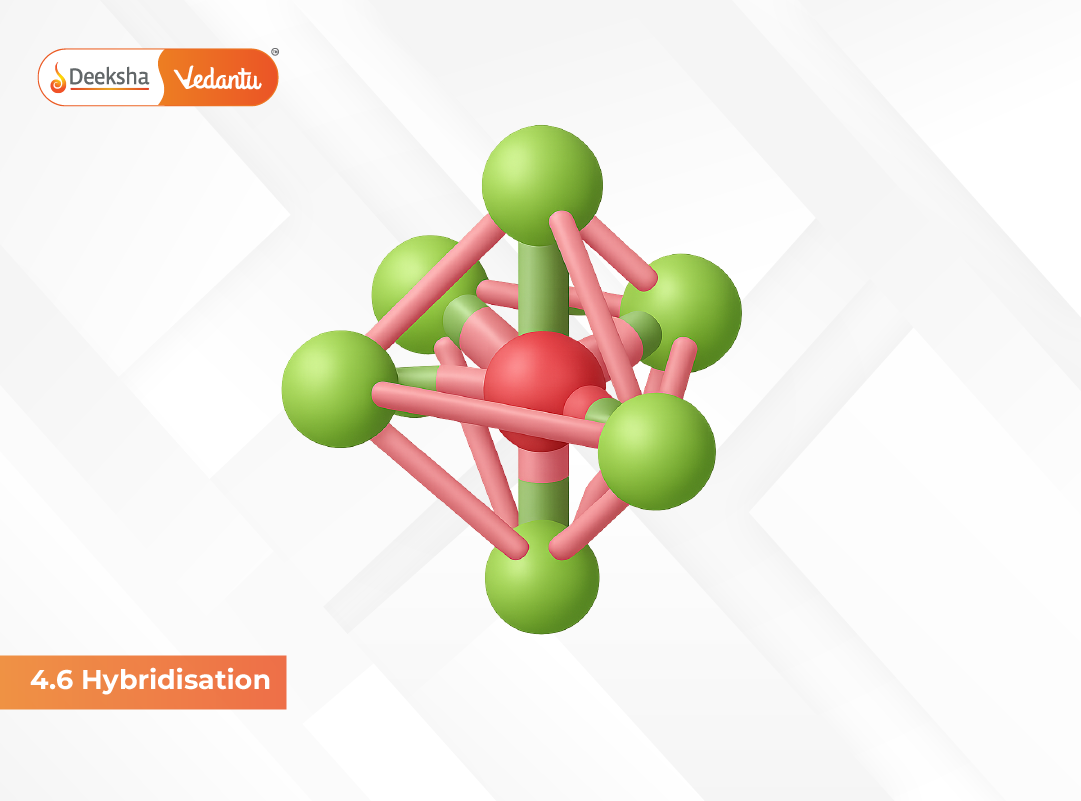
5. sp³d² Hybridization (Octahedral Geometry)
Here, one s orbital, three p orbitals and two d orbitals combine to produce six sp³d² hybrid orbitals, arranged in an octahedral geometry, such as in SF₆.
- Bond angle: 90°
- Shape: Octahedral
- Example molecule: SF₆
Orbital Promotion Process (CH₄, PCl₅, SF₆)
In some molecules, atoms promote electrons to higher orbitals before hybridization to maximise bonding capacity. For example:
- Carbon in CH₄: Ground state (1s² 2s² 2p²) has only two unpaired electrons. One electron from 2s is promoted to 2p, giving four unpaired electrons which undergo sp³ hybridization to form a tetrahedral geometry.
- Phosphorus in PCl₅: Ground state (3s² 3p³) has three unpaired electrons. One electron from 3s is promoted to 3d orbital, allowing formation of sp³d hybrid orbitals.
- Sulphur in SF₆: Ground state (3s² 3p⁴) is promoted to 3s¹ 3p³ 3d², leading to six unpaired electrons and sp³d² hybridization.
Orbital Explanation
To understand hybridization more clearly, it is important to visualise how orbitals mix during bond formation:
- In NH₃, the nitrogen atom undergoes sp³ hybridization. One hybrid orbital contains a lone pair, and the remaining three form N–H sigma bonds, giving a trigonal pyramidal geometry with a bond angle of 107°.
- In H₂O, oxygen undergoes sp³ hybridization, but two of the hybrid orbitals contain lone pairs. Due to the strong repulsion between lone pairs, the H–O–H bond angle is reduced to about 104.5°, resulting in a V-shaped or bent geometry.
- In C₂H₂ (ethyne), carbon atoms undergo sp hybridization to form two sp orbitals. These form sigma bonds between carbon and hydrogen, while the remaining unhybridised p orbitals form two π bonds, creating a triple bond between the carbons.
- In BCl₃, boron undergoes sp² hybridization. Three sp² orbitals form sigma bonds with chlorine, all lying in one plane at 120°.
Examples of Hybridization
| Molecule | Hybridization | Molecular Geometry |
| CH₄ | sp³ | Tetrahedral |
| NH₃ | sp³ + lone pair | Trigonal pyramidal |
| H₂O | sp³ + 2 lone pairs | Bent/V-shaped |
| BCl₃ | sp² | Trigonal planar |
| C₂H₂ | sp | Linear |
These examples demonstrate how hybridization helps predict molecular shapes accurately.
Sigma and Pi Bond Formation (Ethene and Ethyne)
- Ethene (C₂H₄): Each carbon undergoes sp² hybridization. One unhybridised p orbital forms a π bond, resulting in a double bond between the carbons.
- Ethyne (C₂H₂): Each carbon undergoes sp hybridization. Two unhybridised p orbitals form two π bonds (a triple bond). The molecule is linear with a bond angle 180°.
Hybridization Type Table (Atomic Orbitals & Examples)
| Shape/Geometry | Hybridization Type | Atomic Orbitals | Examples |
| Linear | sp | s + p | BeCl₂, C₂H₂ |
| Trigonal planar | sp² | s + 2p | BCl₃ |
| Tetrahedral | sp³ | s + 3p | CH₄, NH₃, H₂O |
| Trigonal bipyramidal | sp³d | s + 3p + d | PCl₅ |
| Octahedral | sp³d² | s + 3p + 2d | SF₆ |
Equatorial and Axial Bonds in PCl₅
In a trigonal bipyramidal structure:
- Three equatorial bonds lie in one plane and are at 120° angles.
- Two axial bonds are perpendicular to the plane and make 90° angles with equatorial bonds.
This explains why axial bonds experience more repulsion and are slightly longer than equatorial bonds.
FAQs
Q1: What is hybridization?
Hybridization is the process of mixing atomic orbitals of nearly equal energy to form new hybrid orbitals that are used to make chemical bonds.
Q2: Who introduced the concept of hybridization?
Linus Pauling introduced the idea of hybridization to explain molecular geometry and bond formation in complex molecules.
Q3: Can hybridization occur without promoting electrons?
Yes, in some cases promotion of electrons is not necessary, but it may still occur to maximise the number of bonds formed.
Q4: Does hybridization affect bond angles?
Yes, the type of hybridization strongly influences bond angles and determines molecular shape. For example, sp³ hybridization leads to 109.5° while sp² leads to 120°.
Q5: Why is hybridization important in NEET and JEE exams?
Hybridization helps students analyse molecular geometry quickly, predict bond formation and understand exceptions where simple theories fail.
Conclusion
Hybridization is a central concept in chemical bonding that explains how atoms form stable, directional and stronger bonds by combining atomic orbitals into hybrid orbitals of equal energy. This theory plays a major role in predicting molecular geometry, bond angles and chemical stability. A thorough understanding of hybridization simplifies the study of molecular structure and helps students excel in Class 11 Chemistry, NEET and JEE competitive exams.





Get Social