In this section, we study in detail how Molecular Orbital Theory explains bonding in homonuclear diatomic molecules such as H₂, He₂, Li₂, Be₂, C₂, N₂, and O₂. Instead of viewing molecular bonding only from the perspective of valence bond theory, MO theory allows us to understand bonding on a quantum mechanical level by considering the combination of atomic orbitals to form molecular orbitals spread over the entire molecule.
These molecular orbitals can be bonding or antibonding, and their relative occupation by electrons directly determines the bond order, stability, bond length, bond dissociation energy, and magnetic nature of a molecule. The molecular orbital energy diagrams, energy ordering of orbitals, bond orders, electronic configurations, and magnetic behavior of these molecules help predict their stability, reactivity, bond strength, and even their existence in nature.
By comparing the electrons in bonding and antibonding orbitals, we can quantitatively determine whether a molecule will form or not, and whether it will be diamagnetic or paramagnetic. This makes MO theory essential not only for NCERT-based understanding but also for NEET and JEE reasoning and numerical questions.
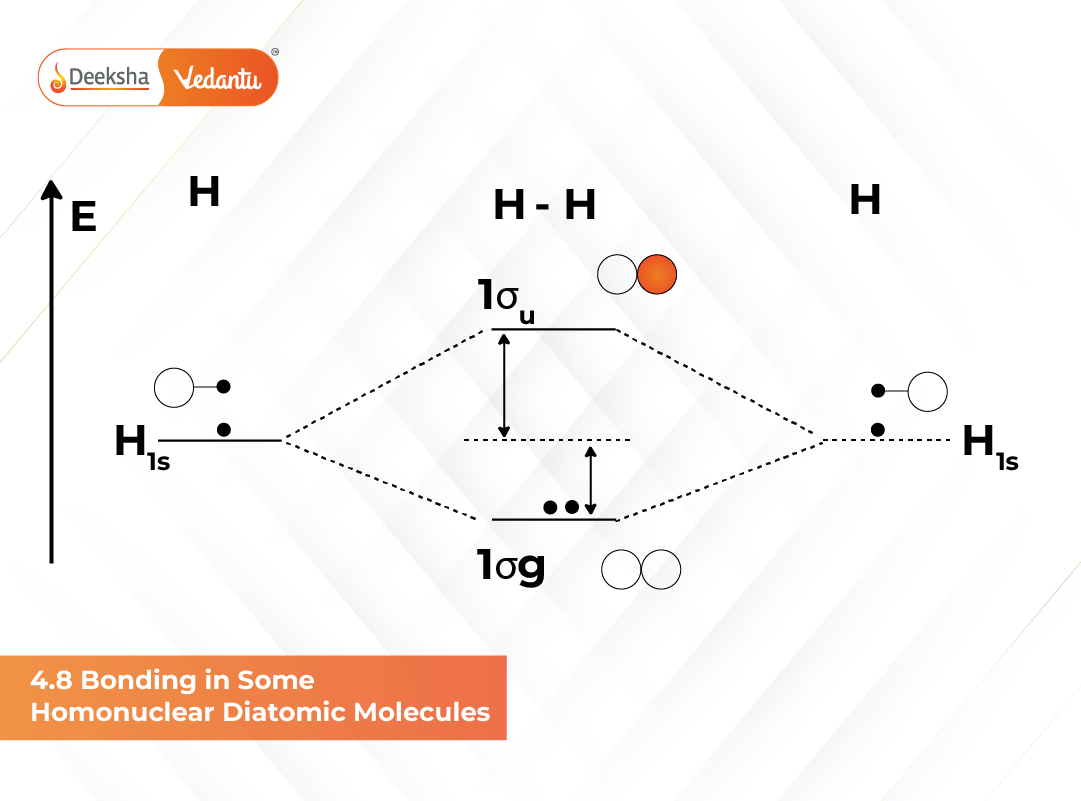
Hydrogen Molecule (H₂)
Hydrogen atoms combine to form H₂. Each hydrogen atom has one electron in the 1s orbital. These electrons fill the bonding σ1s orbital.
Electronic configuration:
H₂ : (σ1s)²
Bond order calculation:
Bond order = ½ (Nb – Na) = ½ (2 – 0) = 1
Thus, H₂ is stable and diamagnetic.
Helium Molecule (He₂)
Each helium atom contains 2 electrons, so He₂ has 4 electrons. These fill both σ1s and σ*1s orbitals.
Electronic configuration:
He₂ : (σ1s)²(σ*1s)²
Bond order = ½ (2 – 2) = 0 → does not exist
He₂ is therefore unstable and not found in nature.
Lithium Molecule (Li₂)
Li has electronic configuration 1s² 2s¹. In Li₂, the valence electrons occupy the σ2s bonding orbital.
Electronic configuration:
Li₂ : (σ2s)²
Bond order = ½ (2 – 0) = 1 → stable
Li₂ is diamagnetic.
Beryllium Molecule (Be₂)
Be has electronic configuration 1s² 2s². In Be₂, antibonding orbitals get filled, reducing stability.
Electronic configuration:
Be₂ : (σ2s)² (σ*2s)²
Bond order = ½ (2 – 2) = 0 → unstable
Be₂ does not exist as a stable molecule.
Carbon Molecule (C₂)
C has 6 electrons. Using modified MO energy order (valid for B₂, C₂, N₂):
σ2s < σ*2s < π2px = π2py < σ2pz
Electronic configuration:
C₂ : (σ2s)² (σ*2s)² (π2px)² (π2py)²
Bond order = ½ (8 – 4) = 2
C₂ is diamagnetic.
Nitrogen Molecule (N₂)
N₂ is one of the most stable diatomic molecules found in nature.
Electronic configuration:
N₂ : (σ2s)² (σ*2s)² (π2px)² (π2py)² (σ2pz)²
Bond order = ½ (10 – 4) = 3
N₂ is stable, has triple bond, and is diamagnetic.
Oxygen Molecule (O₂)
O₂ has 16 electrons. Its energy diagram uses normal MO order:
σ2s < σ2s < σ2pz < π2px = π2py < π2px = π2py < σ2pz
Electronic configuration:
O₂ : (σ2s)² (σ2s)² (σ2pz)² (π2px)² (π2py)² (π2px)¹ (π*2py)¹
Bond order = ½ (10 – 6) = 2
Has 2 unpaired electrons → paramagnetic (proved experimentally).
Summary Table
| Molecule | Bond Order | Stability | Magnetic Nature |
| H₂ | 1 | Stable | Diamagnetic |
| He₂ | 0 | Unstable | — |
| Li₂ | 1 | Stable | Diamagnetic |
| Be₂ | 0 | Unstable | — |
| C₂ | 2 | Stable | Diamagnetic |
| N₂ | 3 | Highly Stable | Diamagnetic |
| O₂ | 2 | Stable | Paramagnetic |
Importance for NEET & JEE
- Explains why O₂ is paramagnetic (cannot be explained by VBT)
- Helps determine bond strength using bond order
- Predicts molecular stability and existence
- Useful in spectroscopy and chemical reactivity
H4 FAQs
Q1: Why does He₂ not exist?
Because its bond order is zero, making it unstable.
Q2: Why is O₂ paramagnetic?
Because it has two unpaired electrons in π* orbitals.
Q3: Which molecule has the highest bond order?
N₂ has a bond order of 3 → strongest bond and least reactivity.
Q4: Why is N₂ chemically inert?
Due to its high bond order and strong triple bond.
Conclusion
Understanding bonding in homonuclear diatomic molecules using Molecular Orbital Theory not only explains stability, magnetic nature, bond order and electronic configuration at a quantum level, but also connects theoretical concepts to real-world chemical behavior. For example, the inertness of N₂ in the atmosphere, the paramagnetic nature of O₂ crucial for respiration, and the non-existence of He₂ all become clear through MO diagrams and bond order calculations. With deeper study, students can relate bond order to bond length, bond energy, chemical reactivity and even spectroscopic data. This topic also forms the foundation for advanced inorganic chemistry, chemical kinetics and coordination chemistry in higher classes. Concepts like anti-bonding orbitals, unpaired electrons and molecular symmetry frequently appear in NEET and JEE questions, especially in reasoning-based or numerical problems.
To further enhance understanding, students should practice drawing MO diagrams, calculating bond orders, predicting magnetism and comparing results with experimental data. Questions often require applying concepts of LCAO, electron promotion, and symmetry of orbitals. Real-life applications include oxygen transport in blood, nitrogen fixation by bacteria and helium’s use in cryogenics due to its non-reactive nature. These examples demonstrate the importance of molecular bonding principles in biology, medicine and industry.
Therefore, mastering MO theory and its applications significantly improves accuracy in exam-based problem solving, enhances analytical thinking and strengthens core understanding of chemical bonding. With consistent practice and visualization of orbital interactions, students can build a strong foundation for competitive exams and higher studies in chemistry.






Get Social