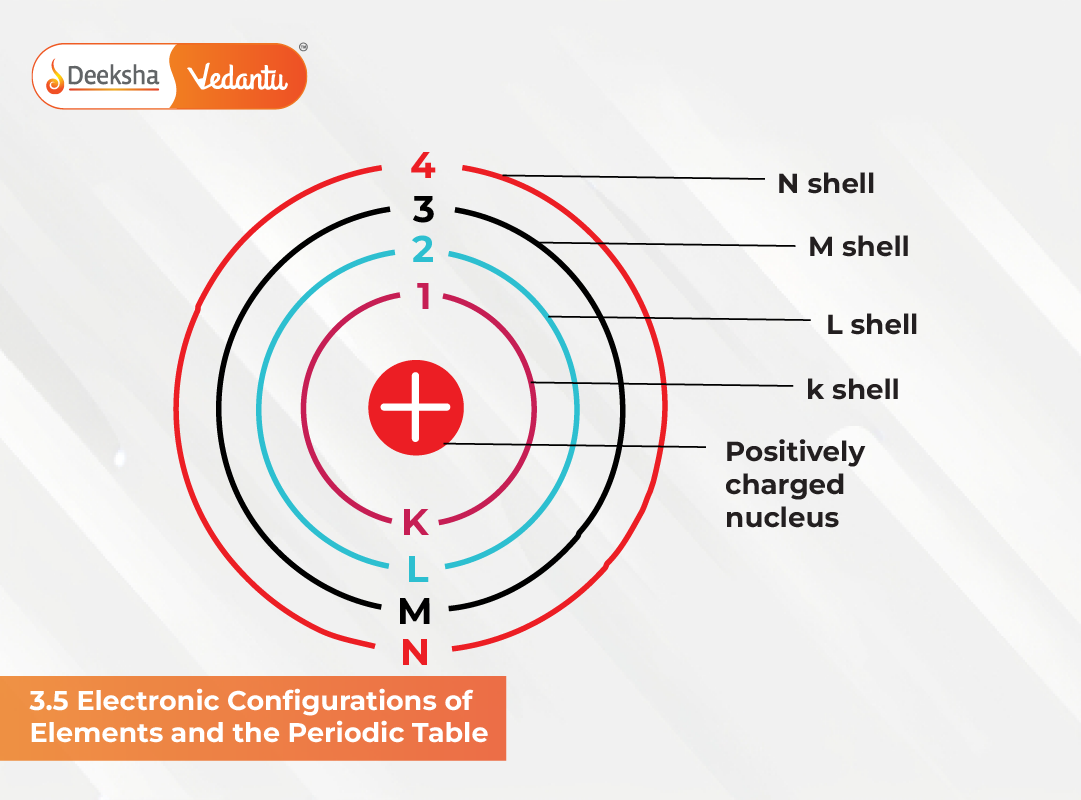
Electronic configurations are the blueprint of atomic structure, and they play a defining role in determining the exact placement of each element in the Periodic Table. Every element’s behaviour—whether chemical or physical—can be traced back to the arrangement of its electrons, especially the outermost ones. By studying these configurations, we gain a deeper understanding of how the table is organised into periods, groups, and blocks, and why certain properties repeat periodically.
The Periodic Table is not just a list of elements; it is a systematic representation of recurring patterns that emerge from electronic arrangements. Whether it is the reactivity of alkali metals, the stability of noble gases, or the versatility of transition elements, all of it is rooted in how electrons occupy orbitals according to quantum rules.
Electronic Configurations in Periods
Periods in the Periodic Table correspond to the filling of principal energy levels (n = 1, 2, 3…). When electrons start occupying a new energy level, a new period begins. The length of each period depends on the number of orbitals available in that shell.
- 1st period (n = 1): Contains only two elements, Hydrogen (1s¹) and Helium (1s²), because the first energy level has only one s-orbital, which can hold a maximum of two electrons.
- 2nd period (n = 2): Includes eight elements as electrons fill the 2s and 2p orbitals. This period introduces p-block elements for the first time.
- 3rd period (n = 3): Also consists of eight elements, following the filling of 3s and 3p orbitals. Even though 3d orbitals exist, they begin filling only in the next period.
- 4th period (n = 4): Contains 18 elements. After the 4s orbitals fill, electrons enter the 3d orbitals, introducing transition metals.
- 5th and 6th periods: Follow similar patterns but include the lanthanoid and actinoid series, dramatically increasing the number of elements.
- 7th period: Involves superheavy elements, many of which are currently under research. Their electronic configurations help scientists predict chemical behaviour even before properties are fully known.
Each period reflects a structured progression of electron filling, demonstrating how atomic size, reactivity, ionisation energies, and other trends evolve across the table.
Groupwise Electronic Configurations
Groups in the Periodic Table align elements with the same number of valence electrons. These electrons dictate chemical reactivity, bonding tendencies, and many observable properties.
| Atomic Number | Element | Electronic Configuration |
| 3 | Li | 1s²2s¹ |
| 11 | Na | 1s²2s²2p⁶3s¹ |
| 19 | K | 1s²2s²2p⁶3s²3p⁶4s¹ |
| 37 | Rb | [Kr]5s¹ |
| 55 | Cs | [Xe]6s¹ |
| 87 | Fr | [Rn]7s¹ |
This consistent pattern shows how alkali metals repeat in behaviour despite belonging to different periods. Larger atomic radii and decreasing ionisation energies down the group all emerge from these predictable configurations.
Blocks of the Periodic Table
The Periodic Table is systematically divided into four blocks, each corresponding to the type of subshell receiving the last electron. This block-wise arrangement is crucial to understanding periodicity.
- s-block: Groups 1 and 2. Valence configurations end in ns¹ or ns². These elements are typically highly reactive metals with simple electron loss tendencies.
- p-block: Groups 13–18. Valence shells end in np¹ to np⁶. This block contains metals, non-metals, and metalloids, making it the most diverse region of the table.
- d-block: Transition metals. Electrons fill the (n−1)d subshells. These elements exhibit variable oxidation states, form coloured ions, and often act as catalysts.
- f-block: Lanthanoids and actinoids. The (n−2)f orbitals fill in this block. These elements show complex electron interactions and unique magnetic and spectral properties.
The concept of blocks is essential because it ties quantum mechanics directly to the macroscopic structure of the Periodic Table.
How Electronic Configuration Determines Position
The placement of an element in the Periodic Table is not arbitrary. It precisely follows the rules of electronic structure:
- The period number corresponds to the highest occupied principal quantum number (n), indicating the outermost energy level.
- The group number is related to the number of valence electrons, which governs chemical reactivity.
- The block identifies the subshell into which the last electron enters, revealing key bonding characteristics.
This relationship explains why atomic structure repeats regularly across the table, forming clear and predictable trends such as:
- Metallic to non-metallic transitions across periods
- Increasing reactivity down alkali metals but decreasing reactivity down halogens
- Gradual changes in atomic and ionic sizes
- Variations in ionisation energies and electronegativities
Understanding electronic configurations allows us to interpret these trends with much greater clarity.
FAQs
Q1. How does electronic configuration determine the position of an element in the Periodic Table?
Electronic configuration identifies both the highest energy level occupied by electrons and the number of valence electrons present. These two factors directly determine the element’s period, group, and block, making its position predictable.
Q2. What is the significance of blocks (s, p, d, f) in the Periodic Table?
Each block represents a specific type of subshell being filled. This categorisation helps in predicting bonding behaviour, reactivity, metallic or non-metallic character, and the types of ions formed.
Q3. Why do 3d orbitals start filling after 4s?
The 4s orbital lies slightly lower in energy than the 3d orbitals in neutral atoms. Therefore, electrons enter 4s first. However, during ionisation, 4s electrons are often removed before 3d electrons due to subtle energy differences.
Q4. What determines the length of each period?
The number of available orbitals in a given principal energy level determines how many elements can occupy that period. For example, n = 3 has nine orbitals, but only the 3s and 3p orbitals fill in Period 3.
Q5. Why are lanthanoids and actinoids placed separately?
These elements involve f-orbital filling and share highly similar chemical properties. To keep the table compact and readable, they are placed below the main body of the Periodic Table.
Conclusion
Electronic configurations reveal the structural logic behind the entire Periodic Table. From the organisation of periods to the alignment of groups and the creation of well-defined blocks, every pattern stems from quantum mechanical principles. By understanding how electrons occupy orbitals, we gain powerful insights into chemical behaviour, periodic trends, and the underlying unity of all elements. This expanded understanding allows students and learners to appreciate the beauty and consistency of the Periodic Table as one of the most important achievements in science.





Get Social