Introduction
The discovery of sub-atomic particles was one of the most groundbreaking achievements in science. It completely changed our perception of matter and laid the foundation for modern physics and chemistry. Before these discoveries, atoms were considered indivisible—the smallest unit of matter as proposed by Dalton. However, late 19th and early 20th-century experiments revealed that atoms themselves are made up of even smaller constituents, each contributing uniquely to the atom’s properties. This discovery not only revolutionized atomic theory but also gave rise to nuclear science, quantum mechanics, and modern chemistry.
The Evolution of the Atomic Concept
John Dalton’s atomic theory was the first scientific attempt to explain the composition of matter. According to Dalton, atoms were solid, indivisible particles that combined in fixed ratios to form compounds. While this model explained the laws of chemical combination, it could not address phenomena like electricity, magnetism, or radioactivity. As scientific instruments improved, researchers began to observe that atoms could emit and respond to electric and magnetic forces—evidence of internal structure. This realization opened the door to the discovery of electrons, protons, and neutrons, three fundamental building blocks of matter.
Discovery of Electrons
The journey toward understanding sub-atomic particles began with the study of electric discharges in gases. When electricity passed through gases at low pressure, scientists noticed mysterious glowing rays. These rays, later known as cathode rays, became the key to identifying the first sub-atomic particle—the electron.
J.J. Thomson’s Cathode Ray Experiment
In 1897, J.J. Thomson used a cathode ray tube (CRT) to conduct a series of experiments. This tube was a sealed glass container with electrodes and a low-pressure gas. When a high voltage was applied, rays moved from the cathode (negative electrode) to the anode (positive electrode). Thomson’s observations were revolutionary:
- The rays moved in straight lines and cast shadows, confirming that they consisted of particles rather than waves.
- The rays were deflected by electric and magnetic fields, proving they carried negative charge.
- Their behavior was independent of the type of gas or electrode material, suggesting they were a universal component of all atoms.
Thomson measured the charge-to-mass ratio (e/m) and discovered that these particles were far lighter than atoms. He concluded that atoms contained smaller negatively charged particles, which he named electrons.
Significance of the Discovery
Thomson’s work fundamentally altered the concept of the atom. His plum pudding model depicted the atom as a sphere of positive charge with embedded electrons—similar to raisins in a pudding. Although later replaced by more accurate models, this was the first representation acknowledging the existence of internal atomic structure.
Discovery of Protons
The identification of the electron posed an immediate question—how could an atom remain neutral if it contained negatively charged electrons? Scientists reasoned that atoms must also include a source of positive charge. This search led to the discovery of protons.
Goldstein’s Canal Ray Experiment
In 1886, E. Goldstein used perforated cathodes in discharge tubes and observed new rays moving in the opposite direction to cathode rays. These were called canal rays or anode rays. The experiments revealed several important points:
- The rays were composed of positively charged particles.
- The charge-to-mass ratio of these particles varied depending on the gas used, indicating that different gases produced different positive ions.
- The lightest and most common positive particle came from hydrogen gas—later named the proton.
Properties and Importance of Protons
- Charge: +1.602 × 10⁻¹⁹ C
- Mass: 1.673 × 10⁻²⁷ kg (approximately 1836 times heavier than an electron)
- Location: Inside the atomic nucleus
Protons define the atomic number (Z) of an element and determine its chemical identity. For example, all atoms with one proton are hydrogen, while those with six are carbon. This discovery was essential to developing the concept of elements and isotopes.
Discovery of Neutrons
Even after the identification of protons and electrons, scientists observed that atomic masses were greater than could be explained by protons alone. This discrepancy hinted at the presence of another neutral particle within the nucleus.
James Chadwick’s Experiment
In 1932, James Chadwick bombarded beryllium with alpha particles. He observed the emission of highly penetrating radiation that was unaffected by electric or magnetic fields, indicating neutrality. Chadwick concluded that these rays consisted of particles with a mass similar to protons but without charge—he named them neutrons.
Properties and Role of Neutrons
- Charge: 0 (neutral)
- Mass: 1.675 × 10⁻²⁷ kg (slightly heavier than a proton)
- Location: Inside the nucleus
Neutrons play a crucial role in stabilizing the nucleus. Without them, the electrostatic repulsion between protons would cause the nucleus to break apart. Neutrons also contribute to isotopic differences and play a vital role in nuclear fission and fusion processes.
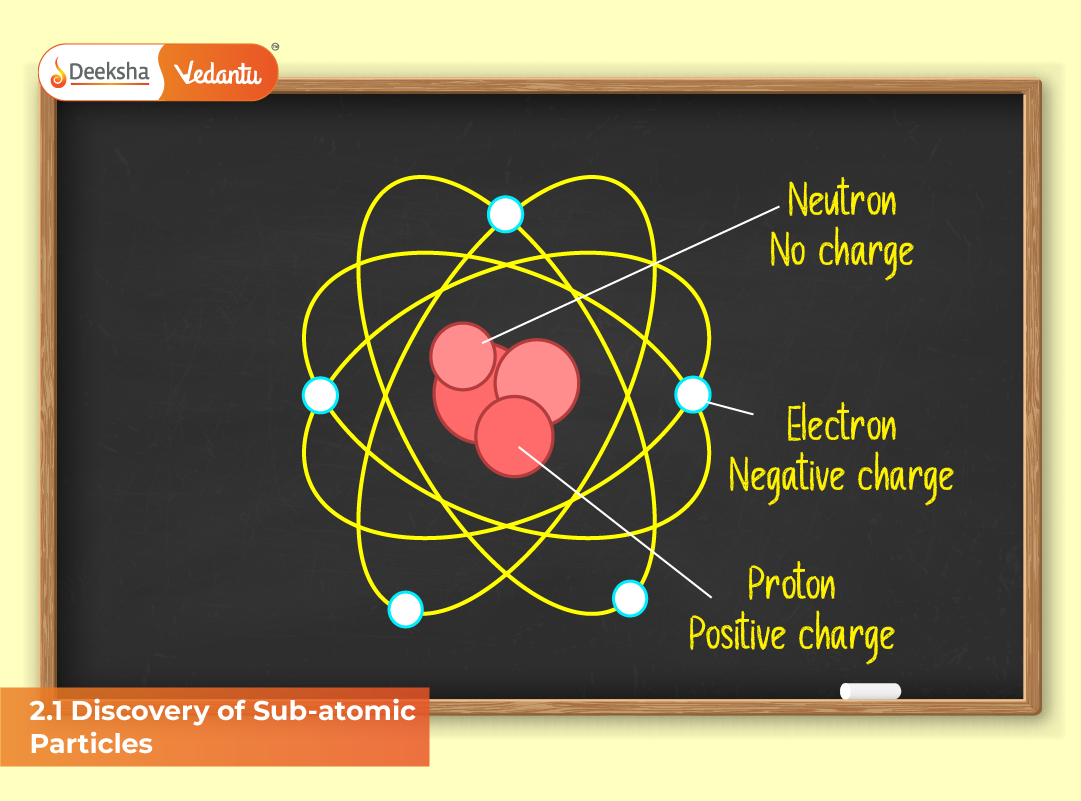
Comparison of Sub-Atomic Particles
| Particle | Symbol | Charge (C) | Mass (kg) | Location |
| Electron | e⁻ | −1.602 × 10⁻¹⁹ | 9.109 × 10⁻³¹ | Orbiting nucleus |
| Proton | p⁺ | +1.602 × 10⁻¹⁹ | 1.673 × 10⁻²⁷ | Inside nucleus |
| Neutron | n⁰ | 0 | 1.675 × 10⁻²⁷ | Inside nucleus |
This table highlights the unique characteristics of each particle. While electrons define chemical reactivity, protons and neutrons determine an atom’s mass and nuclear stability.
The Birth of Modern Atomic Theory
The discovery of sub-atomic particles marked the dawn of a new era in science. The simple, indivisible atom of Dalton’s model evolved into a complex system of charged particles governed by forces and energy interactions. This understanding led to the development of several key theories:
- Rutherford’s Nuclear Model: Proposed that atoms have a dense, positively charged nucleus.
- Bohr’s Model: Introduced quantized electron orbits and explained atomic spectra.
- Quantum Mechanical Model: Described the probability-based nature of electron positions and energies.
Broader Impact on Science
- Chemistry: The electron’s behavior explains bonding and molecular interactions.
- Physics: The discovery of neutrons paved the way for nuclear reactions and isotopic research.
- Technology: The understanding of sub-atomic particles enabled advancements like electron microscopes, nuclear energy, and semiconductor devices.
JEE Focus: What to Know for the Exam
A strong grasp of the discovery timeline and experiments behind electrons, protons, and neutrons is highly testable in JEE. Use the pointers below to revise efficiently:
High-Yield Coverage (JEE Main/Advanced)
- Marks weightage (typical): 2–3 MCQs in JEE Main are common from Atomic Structure basics (including discovery experiments), with 1–2 conceptual items possible in Advanced linked to models/spectra.
- Must-remember facts:
- Electron (Thomson, 1897): cathode rays, e/m determination, nature independent of gas/electrodes.
- Millikan (1909): oil drop experiment → quantized charge 1.602 × 10⁻¹⁹ C.
- Proton (Goldstein, canal rays): positive rays, gas-dependent e/m; hydrogen ion → proton.
- Neutron (Chadwick, 1932): neutral radiation from Be by α-bombardment; knocks out protons from paraffin.
Common JEE Question Types
- Assertion–Reason: “Cathode rays are independent of gas nature” (True) because electrons are universal constituents of atoms (Reason).
- Match the Columns: Map experiment → observation → inference (e.g., Oil drop → integral multiples of charge → charge quantization).
- Numerical: Quick e/m style or charge-counting questions based on Millikan data patterns; simple ratio/comparison rather than heavy computation.
- Conceptual traps:
- Canal rays are not electrons moving backward; they are positive ions moving toward the cathode.
- Cathode rays originate at the cathode and move toward the anode; deflection direction identifies negative charge.
Rapid-Revision Table (Exam Day)
| Item | Key Numbers/Keywords | Takeaway |
| Thomson CRT | e/m; deflection by E and B fields | Electrons exist; universal |
| Millikan | 1.602 × 10⁻¹⁹ C; discrete steps | Charge is quantized |
| Goldstein | Canal (anode) rays; gas-dependent e/m | Positive ions; H⁺ → proton |
| Chadwick | Be + α → neutral radiation | Neutron discovered |
Mini Drills (30–60 sec)
- Why do canal rays bend less than cathode rays in the same fields? Heavier, lower q/m positive ions.
- Which statement is false? Cathode rays depend on electrode material (False; they don’t).
- Millikan droplets showed charges: integral multiples of 1.602 × 10⁻¹⁹ C (True).
FAQs
Q1: How do we know cathode rays are particles and not light?
They cast sharp shadows, spin paddle wheels (mechanical effect), and are deflected by electric and magnetic fields—behaviors inconsistent with neutral light beams.
Q2: Why is the electron’s e/m value important for JEE?
It proves electrons are extremely light and universal, and it underpins many later atomic models and numerical comparisons in exam questions.
Q3: Are canal rays the same for all gases?
No. Their e/m depends on the gas used—canal rays are positive ions of the gas, which is why hydrogen gives the lightest ion (proton).
Q4: What exactly did Millikan quantify in the oil drop experiment?
He measured the elementary charge of the electron and showed all measured droplet charges are integral multiples of 1.602 × 10⁻¹⁹ C.
Q5: How did Chadwick’s experiment prove neutrality of the neutron?
The emitted radiation from Be was unaffected by electric or magnetic fields yet could eject protons from paraffin, implying neutral but massive particles—neutrons.
Q6: What quick mnemonics help remember the timeline?
“TEPN” → Thomson (electron) → e/m → Proton (canal rays) → Neutron (Chadwick). Pair Millikan right after Thomson for charge quantization.
Conclusion
The discovery of sub-atomic particles—electrons, protons, and neutrons—transformed the study of matter. These findings not only shattered the classical notion of indivisible atoms but also built the foundation for all modern physical sciences. From J.J. Thomson’s cathode rays to Chadwick’s identification of the neutron, each step brought humanity closer to understanding the fundamental structure of the universe. Today, our grasp of chemistry, nuclear energy, and particle physics continues to evolve, yet it all traces back to these monumental discoveries that redefined what it means to study the atom.





Get Social