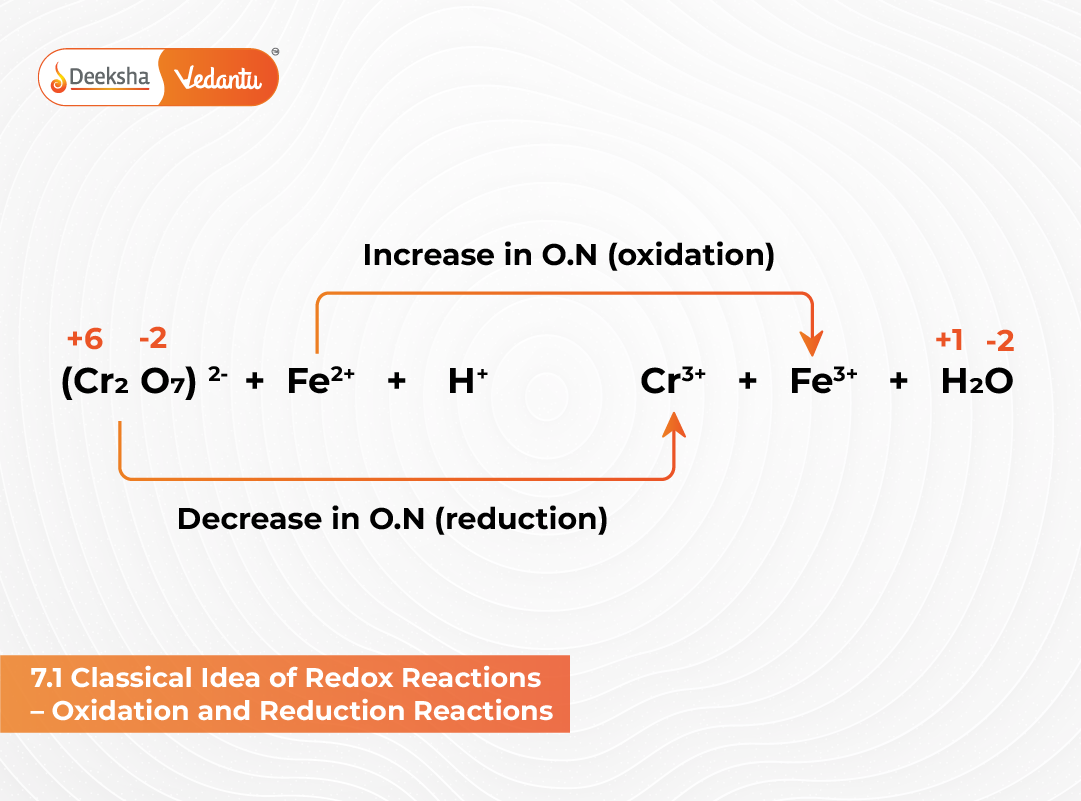
The concept of redox reactions originated from early studies involving the combination of elements with oxygen. Before the modern understanding of electron transfer, chemists defined oxidation and reduction purely in terms of oxygen and hydrogen gain or loss. This classical idea laid the foundation for the modern interpretation of redox processes in chemistry.
Understanding Oxidation
Originally, the term oxidation referred to the addition of oxygen to an element or compound. Because dioxygen is abundant in the atmosphere (~20%), many elements react with it, forming oxides. Such reactions are common on Earth and are fundamental to natural and industrial processes.
Examples:
2Mg(s) + O₂(g) → 2MgO(s)
S(s) + O₂(g) → SO₂(g)
In both cases, the elements magnesium and sulfur combine with oxygen and are said to undergo oxidation. This definition was sufficient for early chemists to describe several reactions, especially those forming oxides or involving combustion.
However, oxidation is not restricted to oxygen addition alone. Reactions involving the removal of hydrogen or the addition of electronegative elements are also considered oxidation processes.
Examples:
CH₄ + 2O₂ → CO₂ + 2H₂O
2H₂S(g) + O₂(g) → 2S(s) + 2H₂O(g)
In both cases, hydrogen atoms are replaced or removed, leading to oxidation.
Understanding Reduction
The reverse of oxidation is reduction, which was originally defined as the removal of oxygen or addition of hydrogen to a substance.
Examples:
2HgO(s) → 2Hg(l) + O₂(g)
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g)
In the first example, mercury(II) oxide loses oxygen to form mercury metal, and in the second, iron(III) oxide loses oxygen and is reduced to metallic iron.
With time, chemists expanded the definition of reduction to include the addition of electropositive elements or the removal of electronegative elements.
Examples:
CH₂=CH₂ + H₂ → CH₃–CH₃ (addition of hydrogen)
2HgCl₂ + SnCl₂ → 2HgCl + SnCl₄ (addition of electropositive element)
Thus, oxidation and reduction were seen as complementary processes — one cannot occur without the other.
Simultaneous Nature of Oxidation and Reduction
Every oxidation reaction is accompanied by a corresponding reduction reaction. When one species gains oxygen (or loses hydrogen), another must lose oxygen (or gain hydrogen). Therefore, oxidation and reduction always occur together.
Example:
H₂S(g) + Cl₂(g) → 2HCl(g) + S(s)
- H₂S loses hydrogen → oxidation
- Cl₂ gains hydrogen → reduction
In this reaction, hydrogen sulfide acts as a reducing agent, while chlorine acts as an oxidizing agent.
Broader Classical Definitions
To generalize oxidation and reduction beyond oxygen and hydrogen transfer, chemists adopted a wider definition based on electronegativity:
- Oxidation: Addition of oxygen or an electronegative element, or removal of hydrogen or an electropositive element.
- Reduction: Addition of hydrogen or an electropositive element, or removal of oxygen or an electronegative element.
This broadened view helped explain reactions like:
Examples:
Mg(s) + F₂(g) → MgF₂(s) (oxidation through addition of electronegative F)
CH₄ + Cl₂ → CH₃Cl + HCl (oxidation by removal of H and addition of Cl)
Similarly, reduction can occur when an element gains an electropositive species or loses an electronegative one.
Example:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
Here, Fe₂O₃ is reduced (loss of oxygen), while CO is oxidized (gain of oxygen).
Oxidation and Reduction Are Interdependent
Since oxidation and reduction are complementary, a single reaction always contains both processes. The substance undergoing oxidation acts as the reducing agent, and the one undergoing reduction acts as the oxidizing agent.
Example 1:
2Na(s) + H₂(g) → 2NaH(s)
Here, sodium loses electrons (oxidized) and hydrogen gains electrons (reduced). Hence, Na is the reducing agent, and H₂ is the oxidizing agent.
Example 2:
2HgO(s) → 2Hg(l) + O₂(g)
Mercuric oxide is reduced to mercury metal by losing oxygen; simultaneously, oxygen is released in molecular form.
Practice Problem
Problem: Identify the species undergoing oxidation and reduction in the following reactions:
(i) H₂S(g) + Cl₂(g) → 2HCl(g) + S(s)
(ii) 3Fe₂O₃(s) + 8Al(s) → 4Al₂O₃(s) + 9Fe(s)
(iii) 2Na(s) + H₂(g) → 2NaH(s)
Solution:
(i) H₂S is oxidized (loss of hydrogen); Cl₂ is reduced (gain of hydrogen).
(ii) Al is oxidized (forms Al₂O₃), Fe₂O₃ is reduced (forms Fe).
(iii) Na is oxidized (forms Na⁺), H₂ is reduced (forms H⁻).
FAQs
Q1. What is the classical idea of oxidation?
In the classical concept, oxidation means the addition of oxygen or the removal of hydrogen from a substance. It does not necessarily involve electron transfer, as explained in the modern definition.
Q2. How is reduction defined in the classical sense?
Reduction refers to the removal of oxygen or the addition of hydrogen or electropositive elements to a substance.
Q3. Why are oxidation and reduction said to be complementary?
Because whenever one substance is oxidized, another is reduced simultaneously. Both processes occur together and cannot happen independently.
Q4. What is the difference between an oxidizing and a reducing agent?
An oxidizing agent reduces itself and causes oxidation of another substance, while a reducing agent oxidizes itself and causes reduction of another substance.
Q5. How did the concept of electronegativity broaden the idea of redox reactions?
The concept of electronegativity allowed oxidation and reduction to be defined in terms of addition or removal of electronegative or electropositive elements, expanding the classical view beyond oxygen and hydrogen transfer.
Q6. Can you give examples of oxidation without oxygen?
Yes, reactions like CH₄ + Cl₂ → CH₃Cl + HCl involve oxidation through removal of hydrogen and addition of chlorine, even though no oxygen is involved.
Q7. What is the modern interpretation of redox reactions?
In modern chemistry, oxidation refers to loss of electrons, and reduction refers to gain of electrons. These definitions apply universally across chemical and electrochemical systems.
Conclusion
The classical concept of oxidation and reduction focuses on oxygen and hydrogen transfer. Although this view is limited, it was foundational for developing the modern electron-transfer concept. This understanding paved the way for electrochemical studies, where oxidation corresponds to electron loss and reduction corresponds to electron gain — the cornerstone of redox chemistry.





Get Social