Calorimetry is a fundamental experimental branch of thermodynamics that deals with the precise measurement of heat transfer during physical or chemical changes. It provides essential insights into the internal energy (ΔU) and enthalpy (ΔH) changes that occur when a reaction takes place. The study of calorimetry bridges theory with practical chemistry by allowing scientists to quantify energy flow within a system and its surroundings.
Understanding Calorimetry
The core concept of calorimetry is based on the law of conservation of energy, which states that energy can neither be created nor destroyed-it can only be transformed from one form to another. When a reaction occurs inside a calorimeter, the heat lost by the system is completely gained by the surroundings and vice versa.
Mathematically, this is represented as:
qₛystem + qsurroundings = 0
which can also be written as:
qₛystem = −qsurroundings
Here, q denotes the amount of heat exchanged. When q is positive, it signifies that the system absorbs heat (an endothermic process). Conversely, a negative q indicates that the system releases heat (an exothermic process). The goal of calorimetry is to measure this heat precisely to understand how energy changes during reactions or phase transitions.
A calorimeter is the instrument used to measure this exchange. It is designed to be thermally insulated, ensuring that no heat is lost to the external environment. Different types of calorimeters exist depending on the conditions of the reaction – primarily constant volume or constant pressure setups.
Measurement of ΔU (Change in Internal Energy)
The change in internal energy, denoted by ΔU, is best measured under constant volume conditions, where no work is done by expansion or compression of gases. This is achieved using a bomb calorimeter, an instrument specifically designed for reactions involving combustion.
Under constant volume, the relationship is simple:
ΔU = qᵥ
Where qᵥ represents the heat absorbed or released when the volume remains unchanged.
Working of a Bomb Calorimeter
A bomb calorimeter consists of a thick-walled steel vessel (the bomb) capable of withstanding high pressures. The sample, often a combustible substance, is placed in the bomb, which is filled with oxygen and immersed in a water bath. The system is perfectly sealed to maintain constant volume.
Steps of operation:
- The sample is weighed accurately and placed in a crucible within the bomb.
- The bomb is charged with oxygen gas at high pressure and sealed.
- It is immersed in a known volume of water inside an insulated calorimeter.
- The reaction is initiated electrically by igniting a fuse wire.
- The heat released raises the temperature of the surrounding water.
- This temperature rise (ΔT) is recorded, and the heat of reaction is calculated.
The heat change is given by:
q = C₍cₐₗ₎ × ΔT
where:
- C₍cₐₗ₎ = heat capacity of the calorimeter
- ΔT = change in temperature
Thus, the internal energy change is:
ΔU = −qᵥ = −C₍cₐₗ₎ × ΔT
The negative sign indicates that the system loses heat, which is gained by the surroundings (water and calorimeter).
Significance of Measuring ΔU
Measuring ΔU helps in determining the internal energy change during combustion, dissolution, and reaction processes. It allows chemists to analyze the energy content of fuels, compare efficiencies, and understand the thermodynamic feasibility of various chemical processes.
Measurement of ΔH (Change in Enthalpy)
The enthalpy change, ΔH, is determined under constant pressure conditions, such as reactions open to the atmosphere. In these cases, the heat exchanged with the surroundings equals the change in enthalpy.
ΔH = qₚ
Where qₚ represents the heat absorbed or released at constant pressure.
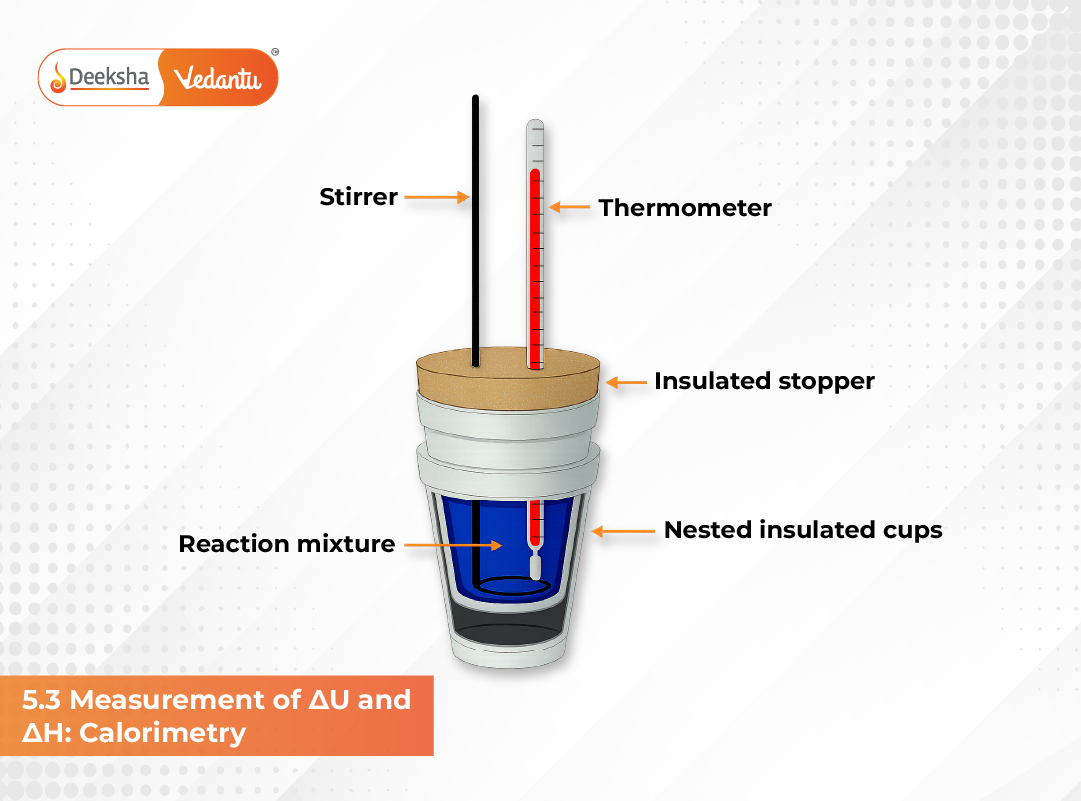
Coffee Cup Calorimeter
A coffee cup calorimeter is a simple and widely used device for measuring heat changes under constant pressure. It consists of two nested Styrofoam cups with a lid, acting as an insulator to prevent heat loss.
Working steps:
- The reaction is carried out in an aqueous solution within the inner cup.
- The temperature change (ΔT) of the solution is measured with a thermometer.
- Assuming minimal heat loss, the heat released or absorbed by the reaction equals the negative of the heat gained by the solution.
The formula used is:
q = m × c × ΔT
Where:
- m = mass of the solution
- c = specific heat capacity of the solvent (usually 4.18 J/g·K for water)
- ΔT = temperature change observed
Thus,
ΔH = qₚ = m × c × ΔT
Importance of Measuring ΔH
Determining ΔH allows scientists to calculate the energy required for chemical reactions such as neutralization, dissolution, and precipitation. It also aids in calculating bond energies and understanding whether a reaction is endothermic or exothermic under atmospheric conditions.
Relationship Between ΔH and ΔU
The enthalpy change (ΔH) and internal energy change (ΔU) are related by the equation:
ΔH = ΔU + PΔV
Here,
- P = pressure
- ΔV = change in volume of the system
This equation is particularly important in reactions involving gases. When there is no change in volume, such as in a bomb calorimeter, ΔH is approximately equal to ΔU. However, for reactions that produce or consume gases, the difference between ΔH and ΔU becomes significant.
For gaseous reactions, the relation can also be written as:
ΔH = ΔU + ΔngRT
Where:
- Δng = change in the number of moles of gas
- R = universal gas constant
- T = absolute temperature in Kelvin
This shows that the enthalpy change accounts for both internal energy change and work done by the system in expanding or contracting against external pressure.
Types of Calorimetry
- Constant Volume Calorimetry: Used to determine ΔU in combustion reactions under sealed conditions.
- Constant Pressure Calorimetry: Used for reactions in open containers to find ΔH.
- Differential Scanning Calorimetry (DSC): Measures differences in energy absorption between a sample and reference during controlled heating.
- Solution Calorimetry: Used for determining heats of dissolution, dilution, or neutralization in liquids.
- Reaction Calorimetry: Employed in industrial processes to monitor exothermic or endothermic reactions in real-time.
Applications of Calorimetry
- Energy Analysis: Determining the calorific value of fuels.
- Biochemistry: Measuring metabolic energy changes.
- Material Science: Studying phase transitions like melting, crystallization, or decomposition.
- Food Industry: Estimating the energy content of food samples.
- Chemical Engineering: Optimizing reactor designs for heat balance and efficiency.
FAQs
Q1. How do ΔU and ΔH differ in measurement and meaning?
ΔU measures internal energy change at constant volume, while ΔH measures enthalpy change at constant pressure. ΔH includes the work associated with volume change (PΔV), whereas ΔU does not.
Q2. What ensures accuracy in a bomb calorimeter experiment?
Accuracy is ensured by maintaining insulation, calibrating the calorimeter using standard substances like benzoic acid, and precisely recording temperature changes.
Q3. Why does a coffee cup calorimeter measure ΔH and not ΔU?
Because it operates at constant atmospheric pressure, it measures the total heat exchanged (qₚ), which directly represents enthalpy change, ΔH.
Q4. What is the significance of calorimeter constant (C₍cₐₗ₎)?
C₍cₐₗ₎ represents the heat capacity of the calorimeter assembly. It is determined through calibration and is crucial for accurate energy measurements.
Q5. How is calorimetry useful in everyday life?
It helps in food labeling for calorie measurement, fuel testing for engines, and understanding energy efficiency in various systems.
Conclusion
Calorimetry serves as a bridge between experimental data and thermodynamic theory, enabling the calculation of ΔU and ΔH – two of the most fundamental parameters in energy studies. Whether through a bomb calorimeter measuring combustion heat or a coffee cup calorimeter tracking solution reactions, this method provides invaluable insights into heat flow, energy conservation, and reaction behavior. By applying calorimetry, scientists and engineers can design safer, more efficient, and energy-conscious systems across multiple fields of science and industry.





Get Social