Understanding bond parameters is essential for predicting the structure, strength, and behavior of chemical compounds. These parameters help in visualizing molecules in three dimensions and play a crucial role while studying chemical bonding. Class 11 NCERT introduces multiple bond parameters such as bond length, bond angle, bond enthalpy, bond order, resonance, and polarity of bonds. These concepts are not only important for board examinations but are also significant for NEET and JEE competitive exams. A detailed understanding of these parameters improves logical reasoning in chemistry and strengthens concepts related to hybridization, VSEPR theory, and molecular orbital theory.
In real-life applications, bond parameters are used in medical research, drug development, nanotechnology, environmental chemistry, industrial material design, and even in space research. Predicting the behavior of molecules depends greatly on understanding how different atoms bond together and how their properties change based on bond strength and geometry.
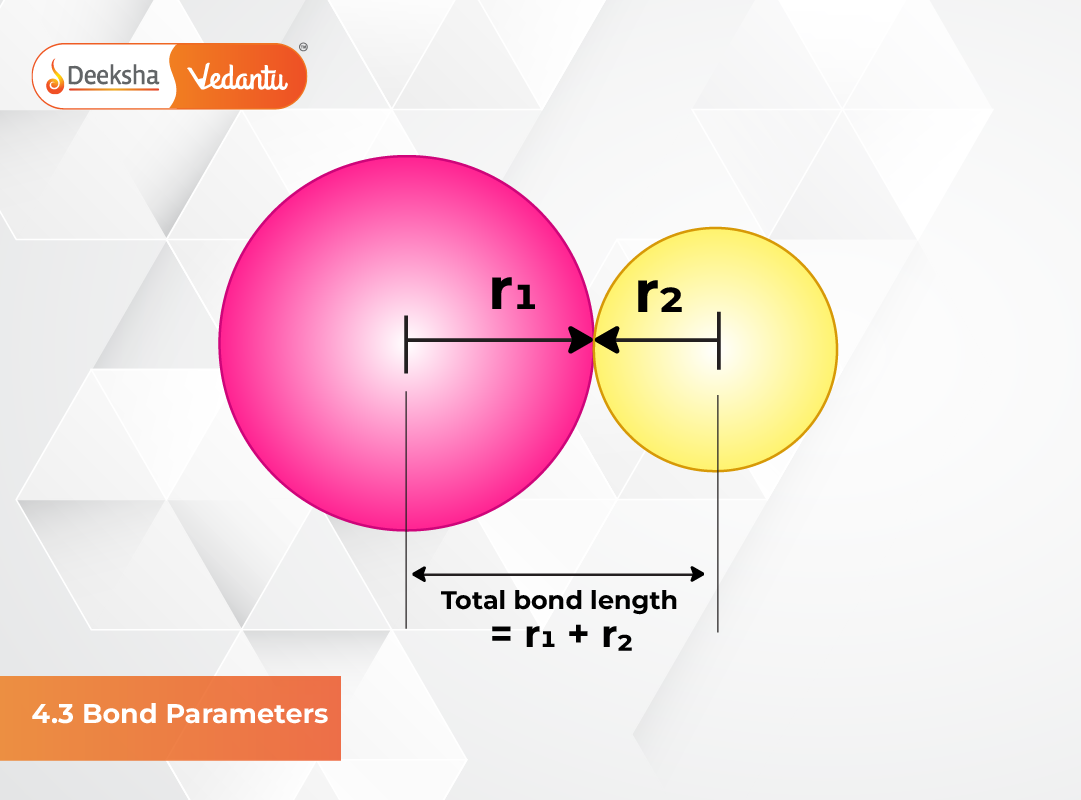
Bond Length
Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule. It represents the strength and stability of the bond. Shorter bond lengths usually indicate stronger bonds, while longer bond lengths represent weaker bonds. Bond lengths are measured using spectroscopic techniques, X-ray diffraction, and electron diffraction methods.
When atoms form a bond, they adjust their positions until the attractive and repulsive forces are balanced. This balancing point becomes the bond length. The bond length also varies depending on atomic size, bond order, and electronegativity difference between atoms.
Covalent Radius
The covalent radius is approximately half of the distance between two similar atoms joined by a covalent bond. It gives an estimate of an atom’s size within a bonding environment. The covalent radius helps predict bond length between different atoms and is an important reference value in theoretical chemistry.
Factors Affecting Bond Length
- Atomic size increases → bond length increases
- Higher bond order → shorter bond length
- Greater electronegativity difference → stronger and shorter bond
- Presence of resonance → bond length becomes intermediate
- Hybridisation → sp < sp² < sp³ (shortest to longest)
Average Bond Lengths for Some Single, Double and Triple Bonds
| Bond Type | Bond Length (pm) |
| H–H | 74 |
| F–F | 142 |
| Cl–Cl | 199 |
| C=C | 134 |
| C≡C | 120 |
| C–O | 143 |
Bond Lengths in Some Common Molecules
| Molecule | Bond Length (pm) |
| H2 (H–H) | 74 |
| F2 (F–F) | 142 |
| Cl2 (Cl–Cl) | 199 |
| N2 (N≡N) | 109 |
| HCl (H–Cl) | 127 |
Bond Angle
Bond angle is the angle between the orbitals containing bonding electron pairs around the central atom. It determines molecular shape, chemical stability, and polarity. For example, in a water molecule (H2O), the bond angle is 104.5°, while in carbon dioxide (CO2), it is 180° due to linear geometry.
Bond angles are explained using VSEPR theory (Valence Shell Electron Pair Repulsion), where electron pairs repel each other and try to stay as far apart as possible.
Factors Affecting Bond Angle
- Lone pair repulsion reduces bond angle
- Higher electronegativity causes bond compression
- Hybridization affects angle: sp³ > sp² > sp
- Multiple bonds increase repulsion and affect geometry
Bond Enthalpy
Bond enthalpy is the energy required to break one mole of bonds of a particular type in gaseous state. Higher bond enthalpy means stronger bonds. Multiple bonds generally have higher bond enthalpies than single bonds. Bond enthalpy helps in calculating reaction enthalpy and predicting reaction feasibility.
Example:
H2(g) → H(g) + H(g) ΔH = 435.8 kJ mol⁻¹
Covalent Radii of Some Elements
| Element | Covalent Radius (pm) |
| H | 37 |
| C | 77 |
| N | 75 |
| O | 73 |
| F | 71 |
| Cl | 99 |
Uses of Bond Enthalpy
- Predicting stability of molecules
- Calculating heat of reactions
- Comparing reactivity of different compounds
- Understanding combustion and biological reactions
Bond Order
Bond order is the number of bonds between two atoms. It indicates bond strength, length, and stability.
- Single bond → Bond order = 1
- Double bond → Bond order = 2
- Triple bond → Bond order = 3
Higher bond order means stronger bond and shorter bond length. For example:
- O₂ → Bond order = 2
- N₂ → Bond order = 3
- O₂⁻ → Bond order = 1.5 (due to resonance)
Modern Understanding – Molecular Orbital Theory
Bond order is also calculated using:
Bond order = (Nb – Na) / 2
Where Nb = number of bonding electrons and Na = number of antibonding electrons.
Resonance Structures
Some molecules cannot be represented by a single Lewis structure. Instead, multiple structures contribute to the actual structure. This phenomenon is called resonance.
Example: Carbonate ion (CO₃²⁻) and carbon dioxide (CO₂) show resonance. The actual structure is a hybrid of all possible forms. Resonance increases stability and equalizes bond lengths.
Effects of Resonance
- Increases molecular stability
- Reduces reactivity
- Leads to equal bond lengths
- Affects polarity and dipole moment
Polarity of Bonds
Polarity arises due to unequal sharing of electrons between atoms. The greater the difference in electronegativity, the higher the polarity. Dipole moment is used to measure polarity.
Dipole Moments of Selected Molecules
| Molecule | Dipole Moment (D) | Geometry |
| HF | 1.91 | Linear |
| HCl | 1.08 | Linear |
| H2O | 1.84 | Bent |
| NH3 | 1.47 | Trigonal-pyramidal |
| CO2 | 0 | Linear |
Importance of Polarity
- Helps determine solubility
- Explains boiling and melting points
- Decides acid-base strength
- Important in drug-receptor interactions
To further understand bond parameters, it is useful to explore how these measurements are used in modern chemistry and molecular design. Bond lengths and angles are critical for predicting molecular geometry, which in turn affects reactivity, polarity, and physical properties such as solubility and boiling point. Computational chemistry often relies on these values to create 3D molecular models that help predict the outcomes of chemical reactions.
Role in Molecular Geometry and Hybridization
Bond parameters also play an important role in hybridization theory. The angle between bonds indicates the type of hybrid orbitals involved in bonding. For instance:
- sp hybridization → bond angle of 180°
- sp² hybridization → bond angle around 120°
- sp³ hybridization → bond angle of 109.5°
These angles arise due to electron repulsion among bonding and non-bonding pairs, as explained by the VSEPR theory.
Factors Affecting Bond Length and Bond Strength
- Greater the bond order → shorter and stronger the bond
- Larger atomic size → longer bond length
- Presence of electronegative atoms → affects bond polarity
- Double and triple bonds → increase bond strength and reduce bond length
Real-Life Applications of Bond Parameters
Bond parameters are used in several scientific fields:
- Drug design: predicting molecular interactions in medicines
- Material science: designing polymers and alloys
- Environmental chemistry: analysing pollutants and gases
- Biochemistry: understanding proteins and enzymes
Comparison Table – Bond Order vs Bond Length
| Bond Order | Typical Bond Length (pm) | Relative Strength |
| Single | 150–200 | Weak |
| Double | 120–140 | Intermediate |
| Triple | 100–120 | Strong |
Advanced Concept – Resonance and Bond Parameters
Resonance structures lead to average bond characteristics. For example, in the carbonate ion (CO₃²⁻), all C–O bonds have equal length due to resonance, resulting in intermediate bond order and greater stability.
FAQs
Q1: What is bond length?
Bond length is the equilibrium distance between the nuclei of bonded atoms and helps determine bond stability.
Q2: What affects bond angle?
Bond angle depends on electron pair repulsion, hybridization, and atomic arrangement.
Q3: What is bond enthalpy?
It is the energy required to break one mole of bonds in gaseous state and helps compare bond strengths.
Q4: Why does resonance occur?
Resonance occurs when no single Lewis structure can explain a molecule completely.
Conclusion
Bond parameters provide the foundation for understanding molecular structure and properties. They connect to VSEPR theory, hybridization, molecular orbital theory, and polarity. From bond length to polarity, each parameter reveals important details about stability, reactivity, and geometry of molecules. A strong understanding of these concepts helps in mastering reaction mechanisms, spectroscopy, and molecular behaviour in biological systems. Mastering these parameters is essential for strong conceptual development in chemistry and competitive exams like NEET and JEE.





Get Social