Alkynes are an important class of unsaturated hydrocarbons that contain at least one carbon–carbon triple bond (C≡C). Represented by the general formula CₙH₂ₙ₋₂, they are more unsaturated than both alkanes and alkenes and show distinct structural and chemical characteristics. The simplest alkyne is ethyne (commonly known as acetylene), widely used in welding, fuel, and organic synthesis.
Nomenclature and Isomerism
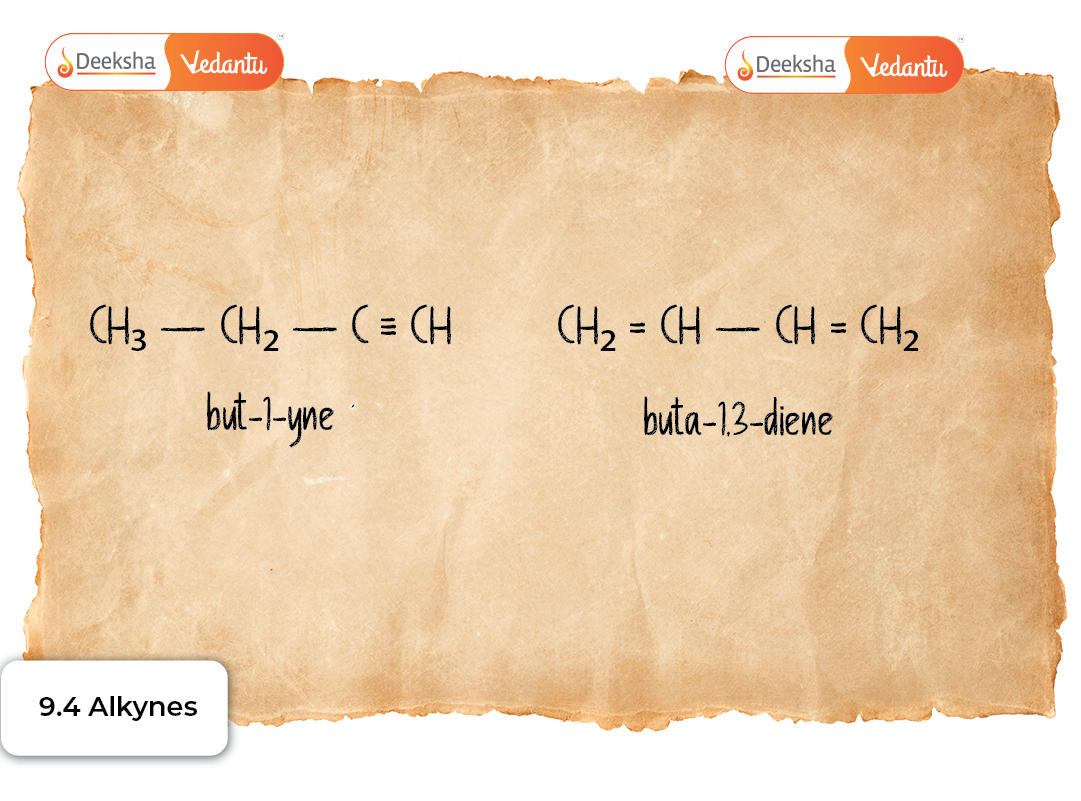
Alkynes are named using the IUPAC system, where the suffix “-yne” indicates the presence of a triple bond.
Rules for Naming
- Identify the longest carbon chain containing the triple bond.
- Number from the end nearest to the triple bond.
- Use the lowest possible locant to denote the position of the triple bond.
- Name substituents and list them alphabetically.
Examples
- CH≡CH → Ethyne
- CH₃–C≡CH → Prop-1-yne
- CH₃–C≡C–CH₃ → But-2-yne
Isomerism
Alkynes exhibit chain isomerism and position isomerism.
- Chain isomerism: Differences in carbon chain branching.
- Position isomerism: Triple bond at different positions.
Geometrical isomerism is not shown by alkynes due to the linear geometry around the triple bond.
Structure of Triple Bond
The carbon atoms in the triple bond are sp hybridised.
Bonding Details
- One sigma (σ) bond is formed by head-on overlap of sp orbitals.
- Two pi (π) bonds are formed by lateral overlap of unhybridised p-orbitals.
Geometry
- Linear geometry
- Bond angle = 180°
- High electron density along the bond axis
This linear arrangement leads to unique reactivity patterns, especially electrophilic addition reactions.
Preparation of Alkynes
NCERT describes several important laboratory and industrial methods for preparing alkynes. Each method relies on either elimination, substitution, or structural transformation to generate the carbon–carbon triple bond.
1. From Dihaloalkanes (Double Dehydrohalogenation)
Vicinal (adjacent carbons) or geminal (same carbon) dihalides undergo two successive elimination reactions to form alkynes.
Stepwise Mechanism:
- First dehydrohalogenation:
- Alcoholic KOH removes one molecule of HX (HBr or HCl).
- Produces a haloalkene.
- Example: CH₂Br–CH₂Br → CH₂=CHBr
- Second dehydrohalogenation:
- Requires a stronger base such as sodium amide (NaNH₂).
- Removes the second HX to form an alkyne.
- CH₂=CHBr → CH≡CH
Overall Reaction:
CH₂Br–CH₂Br + 2KOH → CH≡CH + 2KBr + 2H₂O
Important Notes:
- Vicinal dihalides react more readily than geminal dihalides.
- NaNH₂ is essential for complete conversion to alkynes.
- This is the most widely used laboratory method for alkyne preparation.
2. From Calcium Carbide (Industrial Method)
Calcium carbide reacts with water to produce ethyne.
Reaction:
CaC₂ + 2H₂O → C₂H₂ + Ca(OH)₂
Why This Works:
- CaC₂ contains the carbide ion C₂²⁻, which is protonated on contact with water.
- This generates ethyne instantly.
Applications:
- Cheap industrial production of acetylene.
- Commonly used for oxy-acetylene welding.
3. From Alkenes (Halogenation → Dehydrohalogenation)
Alkenes can be converted to alkynes through a two-step pathway:
Step 1: Halogenation
CH₂=CH₂ + Br₂ → CH₂Br–CH₂Br (vicinal dihalide)
Step 2: Double Dehydrohalogenation
CH₂Br–CH₂Br + alcoholic KOH/NaNH₂ → CH≡CH
Key Points:
- This is an indirect yet efficient route.
- Allows formation of alkynes from readily available alkenes.
- Works well for symmetrical alkenes.
4. Partial Reduction of Higher Alkynes
Higher alkynes can appear as intermediates in controlled reduction reactions of poly-unsaturated hydrocarbons.
Explanation:
When higher polyynes (compounds with multiple triple bonds) undergo selective hydrogenation, individual triple bonds can be isolated to produce alkynes.
Practical Example:
- Controlled hydrogenation of a diyne → monoalkyne.
Although less common in NCERT practice, this concept helps students understand how selective hydrogenation can stop at a triple-bond stage under controlled conditions.
Additional Method Mentioned in Many Texts
Although not emphasised deeply in NCERT, students often encounter an additional method:
5. Dehydrogenation of Alkanes (High Temperature)
At temperatures above 873 K and with chromium/alumina catalysts:
- Alkanes → alkenes → alkynes (stepwise removal of hydrogen)
This is more industrial than academic but helps understand the reactivity ladder:
Alkanes < Alkenes < Alkynes in hydrogen content.
Physical Properties of Alkynes
Alkynes exhibit distinct physical properties due to the presence of the triple bond and their linear geometry. Their physical behaviour follows predictable trends across the homologous series.
1. Physical State
- The first three members-ethyne, propyne, and butyne-exist as colorless gases at room temperature.
- Intermediate alkynes (C₅–C₁₆) are typically volatile liquids.
- Higher alkynes (C₁₇ and above) appear as waxy, low-melting solids.
2. Solubility
- Alkynes are non-polar, so they are insoluble in water.
- They dissolve readily in organic solvents such as ether, benzene, toluene, and chloroform.
- Slightly more soluble than alkanes due to greater polarizability from the triple bond.
3. Density
- Alkynes are lighter than water; their densities range from 0.68 to 0.80 g/mL.
- Density gradually increases with molecular mass.
4. Boiling and Melting Points
- Both increase steadily with an increase in carbon number.
- Alkynes have higher boiling points than alkenes and alkanes of comparable molecular weight because:
- The triple bond increases molecular polarity.
- Stronger London dispersion forces operate.
5. Odour and Appearance
- Lower alkynes have a faint ethereal smell.
- Higher alkynes may have a mild kerosene-like odour.
- Pure ethyne is colorless and odorless, but commercial acetylene has impurities causing smell.
6. Combustion Behaviour
- Alkynes burn with a sooty flame because they have a higher carbon percentage.
- Their combustion is less clean compared to alkanes and alkenes.
7. Bond Strength and Physical Behaviour
- C≡C triple bond is shorter and stronger than C=C and C–C.
- High bond energy influences thermal stability and physical interactions.
Chemical Properties of Alkynes
The chemical behaviour of alkynes is dominated by their carbon–carbon triple bond, which consists of one strong σ bond and two weaker π bonds. The presence of these π bonds makes alkynes more reactive than alkenes toward electrophiles, while the high s-character of the sp-hybridised carbon imparts partial acidity to terminal alkynes. Alkynes undergo a variety of reactions including acid–base reactions, electrophilic additions, oxidative cleavage, metal-ammonia reduction, and polymerisation.
1. Acidic Character
Terminal alkynes (RC≡CH) possess a slightly acidic hydrogen atom. This is a unique property not seen in alkanes or alkenes.
Why are terminal alkynes acidic?
- The carbon bonded to hydrogen is sp-hybridised (50% s-character).
- Higher s-character pulls electron density closer to the carbon nucleus.
- This stabilises the conjugate base (acetylide ion, RC≡C⁻).
- Therefore, pKa ≈ 25, making terminal alkynes weak acids.
Reaction with active metals:
RC≡CH + Na → RC≡C⁻Na⁺ + ½H₂
Importance of acetylide ions:
Acetylide ions act as strong nucleophiles useful in:
- Chain extension reactions
- Nucleophilic substitution (SN2)
- Formation of longer carbon skeletons
Distinguishing terminal vs internal alkynes:
Only terminal alkynes react with Na or Ag⁺/NH₃ to form precipitates (AgC≡CR), aiding identification.
2. Electrophilic Addition
Alkynes undergo electrophilic addition because the π electrons are exposed and easily attacked.
(a) Hydrogenation (H₂ addition)
- Catalysts: Pt, Pd, Ni
- Complete hydrogenation → Alkane
- Controlled hydrogenation with Lindlar catalyst → cis-alkene
- Sodium in liquid ammonia reduces alkynes to trans-alkenes
(b) Halogenation (Cl₂, Br₂)
- One equivalent → dihaloalkene
- Excess halogen → tetrahalide
Example:
HC≡CH + Br₂ → CHBr=CHBr → CHBr₂–CHBr₂
(c) Hydrohalogenation (HX addition)
- First HX addition gives haloalkene.
- Second HX addition forms geminal dihalide.
- Markovnikov addition occurs in unsymmetrical alkynes.
- No peroxide effect is observed with alkynes (unlike alkenes).
(d) Hydration (H₂O addition)
- Requires H₂SO₄ + HgSO₄ catalyst.
- Forms enol, which rapidly tautomerises to ketone.
Example:
CH≡CH → CH₂=CHOH → CH₃CHO (acetaldehyde)
3. Oxidation
The products depend on the strength of the oxidising agent.
(a) Cold, dilute KMnO₄
- Converts alkynes into 1,2-diketones.
- Example: HC≡CH → OHC–COH (glyoxal)
(b) Hot alkaline KMnO₄ or acidic KMnO₄
- Causes complete cleavage of the triple bond.
- Produces carboxylic acids.
- Reaction is similar to oxidative cleavage of alkenes but more vigorous.
Example:
RC≡CR → RCOOH + RCOOH
4. Ozonolysis
Alkynes react with ozone to form ozonides which, on hydrolysis, yield:
- Carboxylic acids (most cases)
- Carbon dioxide (when terminal C is involved)
This reaction is widely used for structure elucidation.
Example:
HC≡CH → O₃ → HC(O)OH + HC(O)OH
5. Polymerisation
Under specific catalytic conditions, alkynes undergo polymerisation.
(a) Linear polymerisation
Large polymer chains such as polyacetylenes are formed.
(b) Cyclotrimerisation
Three molecules of ethyne combine to form benzene.
3 HC≡CH → C₆H₆
(c) Industrial significance
- Polyacetylenes used in electrical conductivity applications
- Cyclotrimerisation used in aromatic ring synthesis
FAQs
Q1. Why are terminal alkynes acidic?
Because the sp hybridised carbon holds the hydrogen more tightly, allowing deprotonation.
Q2. Do alkynes show geometrical isomerism?
No, due to their linear geometry.
Q3. What reagent converts dihalides into alkynes?
Alcoholic KOH followed by sodium amide (NaNH₂).
Q4. Which gas is produced when calcium carbide reacts with water?
Ethyne (acetylene).
Q5. What happens during ozonolysis of alkynes?
They form carboxylic acids.
Conclusion
Alkynes are highly reactive unsaturated hydrocarbons that play an essential role in organic synthesis and industry. Their unique triple bond structure, acidic character, and broad range of reactions make them an important topic in the study of hydrocarbons. At Deeksha Vedantu, we guide students through these concepts with clarity to strengthen their foundational understanding.





Get Social