What is Bohr’s Model of an Atom?
The Bohr model, proposed by Niels Bohr in 1915, is a modification of Rutherford’s atomic model. Rutherford’s model described an atom as a nucleus (positively charged) surrounded by electrons (negatively charged). Bohr improved this by explaining that electrons move in fixed orbits (shells) around the nucleus, with each orbit having a specific energy level.
Introduction to the Bohr Model
Bohr’s theory introduced the idea that electrons travel in specific paths, or orbits, around the nucleus and that these orbits have fixed energy levels. He built upon Rutherford’s model by detailing how these energy levels work.
- The nucleus is positively charged.
- Electrons move in fixed orbits around the nucleus.
- Electrons further from the nucleus have more energy, and those closer have less energy.
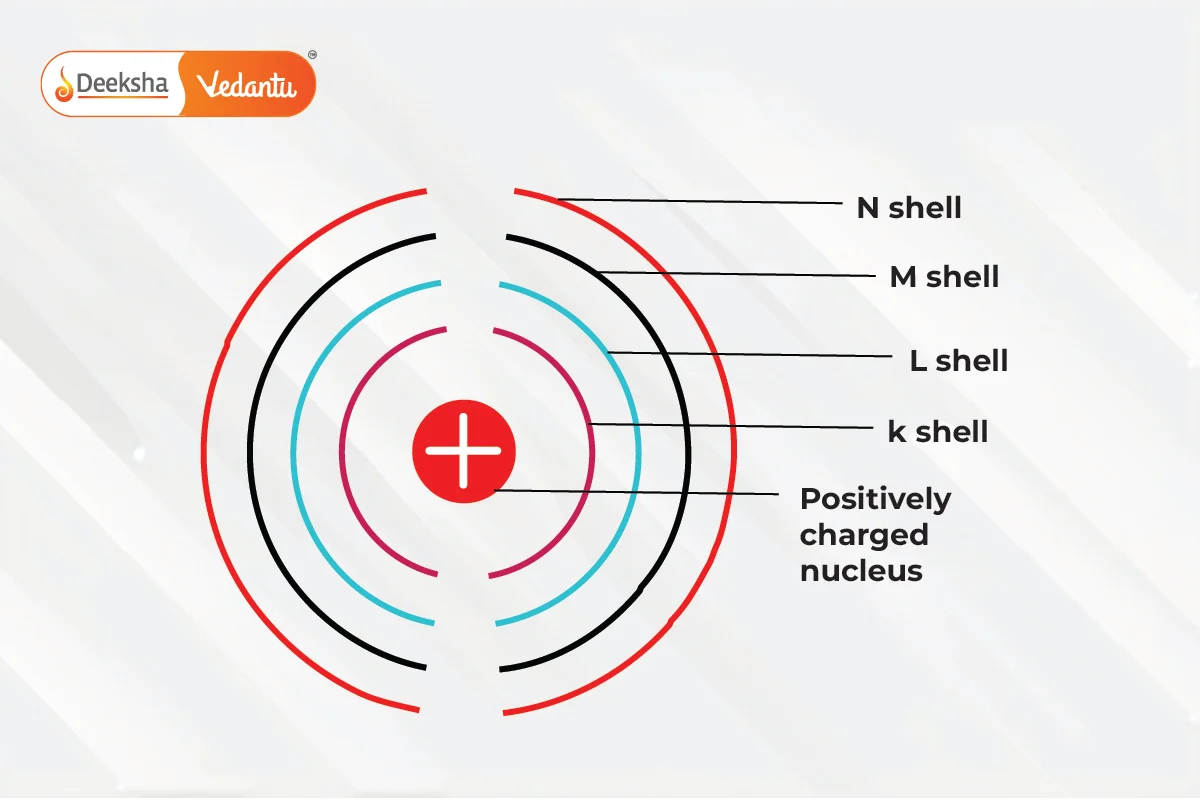
In Bohr’s model:
- The nucleus is positively charged.
- Electrons move in fixed orbits around the nucleus.
- Electrons further from the nucleus have more energy, and those closer have less energy.
Postulates of Bohr’s Model
- Electrons in Orbits: Electrons revolve around the nucleus in definite paths called orbits or shells.
- Fixed Energy Levels: Each orbit has a fixed energy level and is known as an orbital shell.
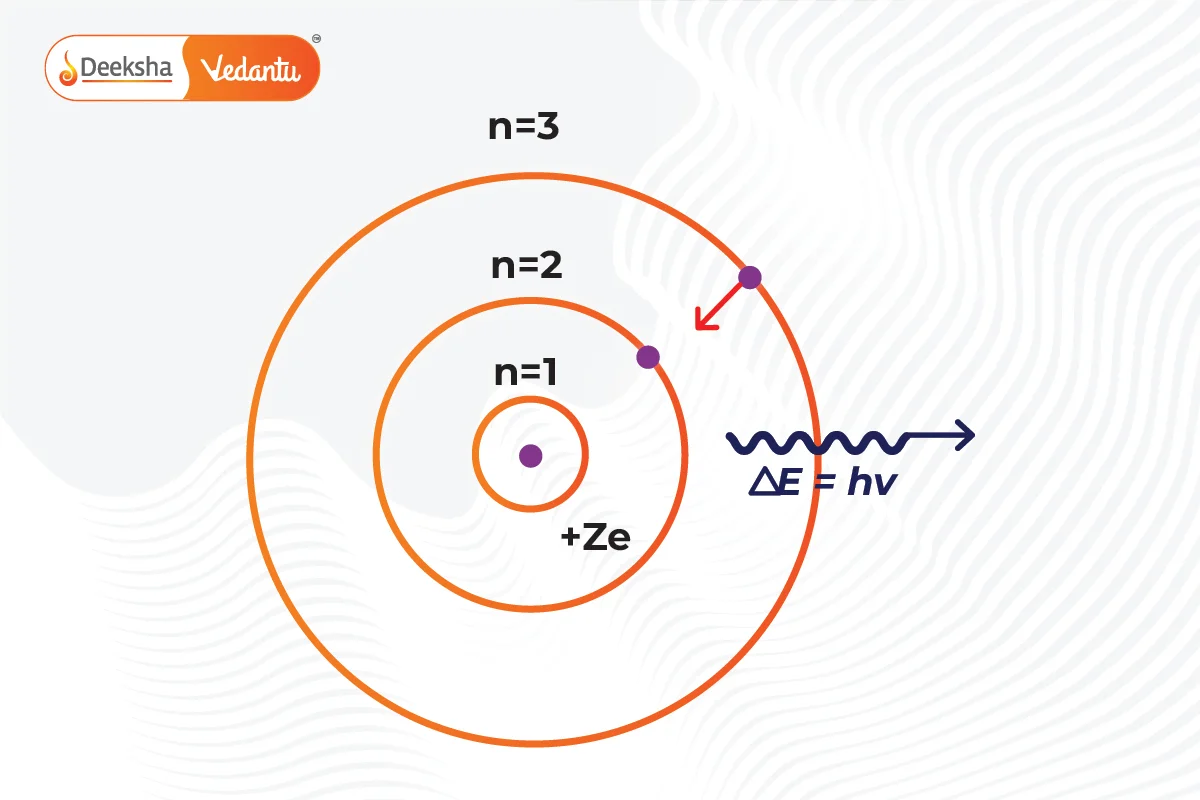
- Quantum Numbers: Energy levels are denoted by integers (n=1, 2, 3…), starting from the closest to the nucleus (n=1) with the lowest energy level. These are labeled as K, L, M, N… shells. When an electron is at the lowest energy level, it’s in the ground state.
- Energy Absorption and Emission: Electrons can move to a higher energy level by absorbing energy and drop to a lower energy level by releasing energy.
Limitations of Bohr’s Model
- Zeeman Effect: Bohr’s model could not explain the splitting of atomic spectra in a magnetic field (Zeeman Effect).
- Stark Effect: It also failed to explain the splitting of atomic spectra in an electric field (Stark Effect).
- Heisenberg Uncertainty Principle: The model violates this principle, which states that the position and momentum of an electron cannot both be precisely determined.
- Larger Atoms: Bohr’s model couldn’t accurately describe the spectra of larger atoms with more electrons.
FAQs
Bohr’s model was eventually replaced by the quantum mechanical model, which provides a more accurate and comprehensive understanding of electron behavior and atomic structure, accounting for the principles of quantum mechanics and the Heisenberg Uncertainty Principle.
Bohr’s model is based on a simple system with one electron (like hydrogen). Larger atoms have more complex electron interactions and energy levels, which Bohr’s model could not accurately describe.
Bohr’s model explained atomic stability by proposing that electrons move in fixed orbits with specific energy levels, preventing them from spiraling into the nucleus due to electrostatic attraction.
Electrons can move from a lower to a higher energy level by absorbing energy. Conversely, they can move from a higher to a lower energy level by releasing energy. This absorption or emission of energy often results in the emission of light at specific wavelengths, forming an atomic spectrum.
Quantum numbers in Bohr’s model represent the energy levels of the orbits around the nucleus. The number (n) indicates the orbit’s distance from the nucleus and its energy level, with n=1 being the closest and lowest energy level.
Rutherford’s model described the atom with a central nucleus and electrons around it but did not explain how electrons are arranged. Bohr introduced the concept of fixed orbits with specific energy levels, providing a clearer structure for the arrangement of electrons.
Bohr’s model, proposed by Niels Bohr in 1915, describes an atom with a positively charged nucleus surrounded by electrons moving in fixed orbits (shells) around it. Each orbit has a specific energy level.






Get Social