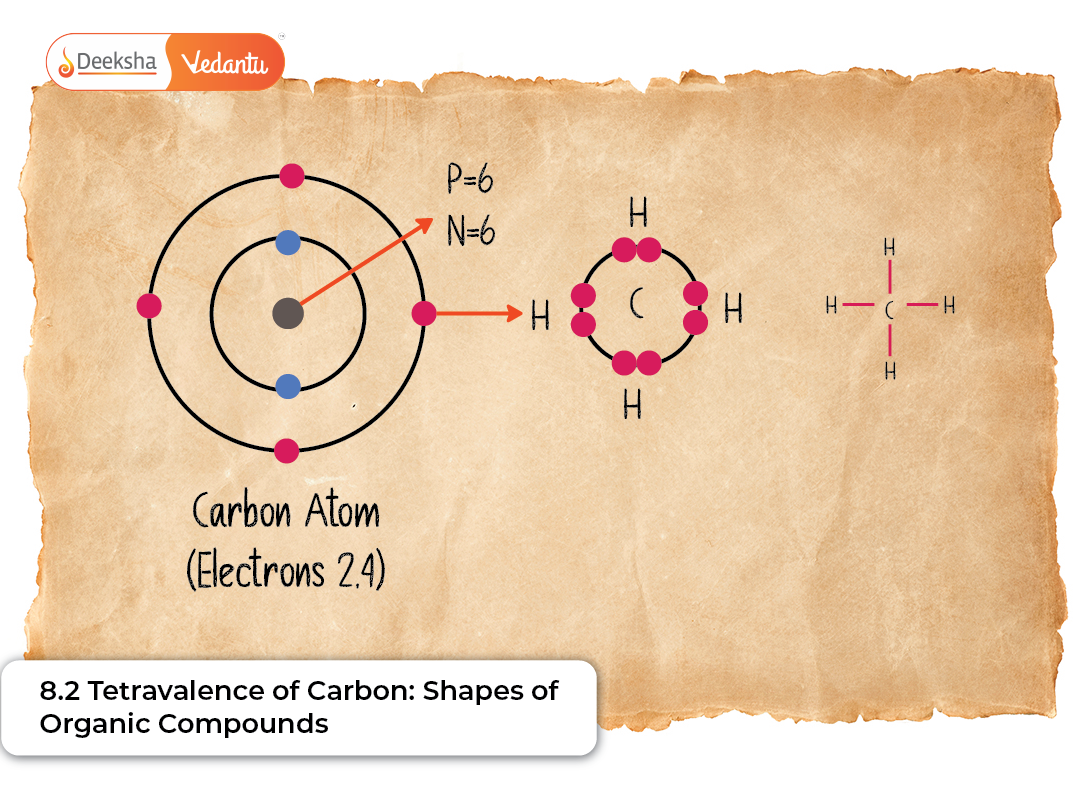
The concept of tetravalence is one of the most fundamental ideas in organic chemistry. Carbon’s ability to form four covalent bonds is the reason behind the existence of millions of organic compounds, from the simplest hydrocarbons to the most complex biomolecules found in living organisms. The arrangement of these bonds in space gives rise to molecular shapes, which in turn influences the chemical and physical properties of organic substances.
Understanding the connection between tetravalence, hybridisation, and molecular geometry is crucial for mastering organic chemistry. At Deeksha Vedantu, we guide students through these concepts using structured explanations, visualisation techniques, and real-life analogies so they can confidently approach advanced topics like stereochemistry, resonance, and reaction mechanisms.
Understanding Tetravalence of Carbon
Carbon has an atomic number of 6 and an electronic configuration of 1s² 2s² 2p². In its ground state, carbon has only two unpaired electrons in the 2p orbital, which would allow the formation of only two covalent bonds. However, carbon undergoes promotion of an electron from the 2s orbital to the empty 2p orbital, resulting in four unpaired electrons. This enables the formation of four strong covalent bonds, a characteristic known as tetravalency.
Tetravalency makes carbon versatile enough to form:
- Four single bonds (as in methane)
- Two double bonds (as in carbon dioxide)
- One double bond and two single bonds (as seen in many organic molecules)
- One triple bond and one single bond (as in alkynes)
Because these combinations are stable and diverse, carbon chemistry forms the basis of organic life and synthetic materials.
Why Tetravalency Leads to Diversity
Carbon’s four-bond capacity allows it to combine with various elements such as hydrogen, oxygen, nitrogen, sulfur, halogens, and even metals. It also allows carbon atoms to bond with one another repeatedly, forming long chains, branched structures, and ring systems. This property, called catenation, is stronger for carbon than for any other element.
At Deeksha Vedantu, students learn how carbon’s bonding capacity affects the behaviour and stability of molecules using structural models and bond energy comparisons.
Hybridisation: The Key to Molecular Shapes
Hybridisation is a concept proposed to explain the geometry of molecular bonding. In this process, atomic orbitals mix to form new hybrid orbitals, each with the same energy level. These hybrid orbitals determine the bond angles and shape of the molecule.
Understanding hybridisation is essential for predicting:
- Bond angles
- Molecular geometry
- Polarity
- Shape-dependent chemical reactivity
- Stereochemical outcomes of reactions
Let’s explore the three main types of hybridisation in carbon.
sp³ Hybridisation – Tetrahedral Geometry
In sp³ hybridisation, carbon mixes one s orbital and three p orbitals to form four equivalent sp³ hybrid orbitals. These orbitals arrange themselves as far apart as possible to minimise electron pair repulsion, creating a tetrahedral shape.
Key features:
- Bond angle: 109.5°
- Structure: Tetrahedral
- Example: Methane (CH₄)
- Characteristics: Forms single bonds (σ-bonds), leading to stable, less reactive molecules
Tetrahedral geometry is extremely important because it forms the backbone of saturated hydrocarbons (alkanes) and many biomolecules.
At Deeksha Vedantu, students use 3D molecular tools that show how tetrahedral angles give rise to chirality and other stereochemical effects.
sp² Hybridisation – Trigonal Planar Geometry
In sp² hybridisation, carbon mixes one s orbital and two p orbitals, forming three sp² hybrid orbitals arranged in a trigonal planar shape.
Characteristics:
- Bond angle: 120°
- Structure: Trigonal planar
- Example: Ethene (C₂H₄)
- Additional feature: Contains a double bond consisting of one sigma bond and one pi bond
Because the pi bond restricts rotation, molecules with sp² hybrid carbon atoms often exhibit geometric isomerism (cis/trans).
Students learn how the rigidity of double bonds influences shape, reactivity, and stereochemistry.
sp Hybridisation – Linear Geometry
In sp hybridisation, carbon mixes one s orbital and one p orbital to create two sp hybrid orbitals arranged in a straight line. This leads to a linear molecular shape.
Properties:
- Bond angle: 180°
- Structure: Linear
- Example: Ethyne (C₂H₂)
- Features: Contains a triple bond (one sigma and two pi bonds)
Alkynes with triple bonds are more reactive due to their electron-rich pi bonds and shorter, stronger carbon-carbon bonds.
At Deeksha Vedantu, we break down triple-bond reactivity using simple electron flow diagrams to help students visualise reaction pathways.
How Shape Influences Properties
The geometry of organic molecules significantly impacts their physical and chemical behaviour. Important effects include:
Polarity and Solubility
The symmetry of the molecule determines whether it is polar or nonpolar. For example:
- Methane is nonpolar due to its perfect tetrahedral symmetry.
- Alcohols may be polar because of their bent O–H bond arrangement.
Polarity influences solubility, boiling point, and interactions with biological systems.
Reactivity Patterns
Double and triple bonds create electron-rich regions that attract electrophiles. Meanwhile, tetrahedral single-bonded carbons tend to be less reactive.
Intermolecular Forces
Molecular geometry affects the strength of forces like hydrogen bonding, dipole interactions, and van der Waals forces, which determine boiling and melting points.
Biological Recognition
Enzymes and receptors in living organisms recognise molecules based on shape. Even small changes in geometry can dramatically affect biological activity.
Why Tetravalence Matters in Organic Chemistry
Tetravalence is the defining feature of carbon and the reason organic chemistry exists as a separate branch. Without tetravalence, carbon could not form:
- Long-chain hydrocarbons
- Aromatic rings
- Complex branching structures
- Functional group diversity
This ability enables carbon to form the structural framework of life. DNA, proteins, carbohydrates, fats, vitamins, and hormones all rely on the tetravalence of carbon.
Real-World Examples of Molecular Shapes
Methane (CH₄)
Methane is the simplest organic molecule containing carbon and is a perfect example of sp³ hybridisation. Its four C–H bonds are arranged symmetrically in a tetrahedral shape with bond angles of 109.5°. This symmetry makes methane nonpolar, influencing its behaviour as a clean-burning fuel widely used in households and industries. Its geometry also helps students understand how tetrahedral structures serve as the building block for larger alkanes.
Ethanol (C₂H₅OH)
Ethanol contains carbon atoms mainly in the sp³ hybridised state, giving it a tetrahedral geometry around each carbon. Its O–H group is highly polar, enabling hydrogen bonding with water. This explains why ethanol dissolves easily in water and is used in medicines, sanitizers, and alcoholic beverages. The geometry also affects its boiling point, evaporation rate, and interaction with biological systems. At Deeksha Vedantu, we relate these properties to real-life applications so students see the connection between structure and function.
Benzene (C₆H₆)
Benzene is a planar molecule where each carbon is sp² hybridised. The ring structure contains delocalised π-electrons, giving benzene unique stability known as aromaticity. Its flat, hexagonal geometry influences how it undergoes substitution reactions rather than addition reactions. Benzene is a foundational structure in aromatic chemistry and appears in dyes, pharmaceuticals, plastics, and industrial solvents. By visualising its planar shape, students better understand resonance and electron delocalisation.
Acetylene (C₂H₂)
Acetylene, or ethyne, is a linear molecule with sp hybridisation, resulting in a straight 180° bond angle. Its carbon atoms are connected by a triple bond, which stores a large amount of energy. When burned with oxygen, acetylene produces an extremely hot flame, making it useful in welding and metal cutting. This example shows how molecular geometry can directly determine industrial usefulness.
These examples help students see how molecular geometry influences polarity, reactivity, industrial applications, solubility patterns, and biological interactions. Understanding these real-world connections strengthens conceptual clarity and prepares learners for deeper organic chemistry topics.
FAQs
Q1. What does tetravalence of carbon mean?
It means carbon can form four strong covalent bonds due to its four valence electrons.
Q2. Why does carbon form different shapes?
Different hybridisation states (sp³, sp², sp) create different molecular geometries and bond angles.
Q3. How does hybridisation affect reactivity?
sp-hybridised carbons in triple bonds are more reactive than sp² and sp³ hybridised carbons because of higher s-character.
Q4. Why is methane tetrahedral in shape?
Because carbon undergoes sp³ hybridisation, forming four identical bonds arranged at 109.5°.
Q5. Can carbon form more than four bonds?
Normally no, but in some advanced inorganic compounds, carbon can expand its bonding beyond four by forming coordinate bonds.
Conclusion
Carbon’s tetravalence and hybridisation together form the structural foundation of organic chemistry. By understanding how orbitals combine and how shapes emerge from hybridisation, students gain deeper insight into molecule stability, reactivity, and behaviour. At Deeksha Vedantu, we simplify these ideas through visual learning, detailed explanations, and continuous practice, ensuring students are fully prepared for upcoming concepts such as stereochemistry, isomerism, and reaction mechanisms.










Get Social