Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It enables chemists to predict how much product can be formed from given amounts of reactants or how much reactant is required to produce a desired quantity of product. Derived from balanced chemical equations, stoichiometry serves as the mathematical link between theoretical predictions and real-world laboratory measurements. In JEE and other entrance examinations, stoichiometry is among the most fundamental topics as it builds the base for solving numerical problems related to reaction yield, molecular relationships, and limiting reagents.
Stoichiometric calculations typically involve converting quantities of substances between moles, masses, and volumes using balanced chemical equations. These calculations rely heavily on the mole concept, molar mass, and Avogadro’s number, forming the bridge between microscopic atomic behavior and macroscopic measurable quantities.
Key Concepts of Stoichiometry
- Balanced Chemical Equation: The equation must first be balanced to ensure conservation of mass, meaning the number of atoms of each element remains constant on both sides of the reaction.
- Mole Ratio: Derived from the coefficients of a balanced equation, the mole ratio indicates the exact proportion in which reactants combine and products form.
- Molar Mass: Expressed in grams per mole (g mol⁻¹), it connects the microscopic concept of moles with the measurable quantity of mass.
- Conservation of Mass: A cornerstone of chemistry, it states that the total mass of the reactants equals the total mass of the products.
- Volume Relationships (Gaseous Reactions): For reactions involving gases, the volumes of reactants and products can also be related using Avogadro’s law at constant temperature and pressure.
Example of a Stoichiometric Calculation
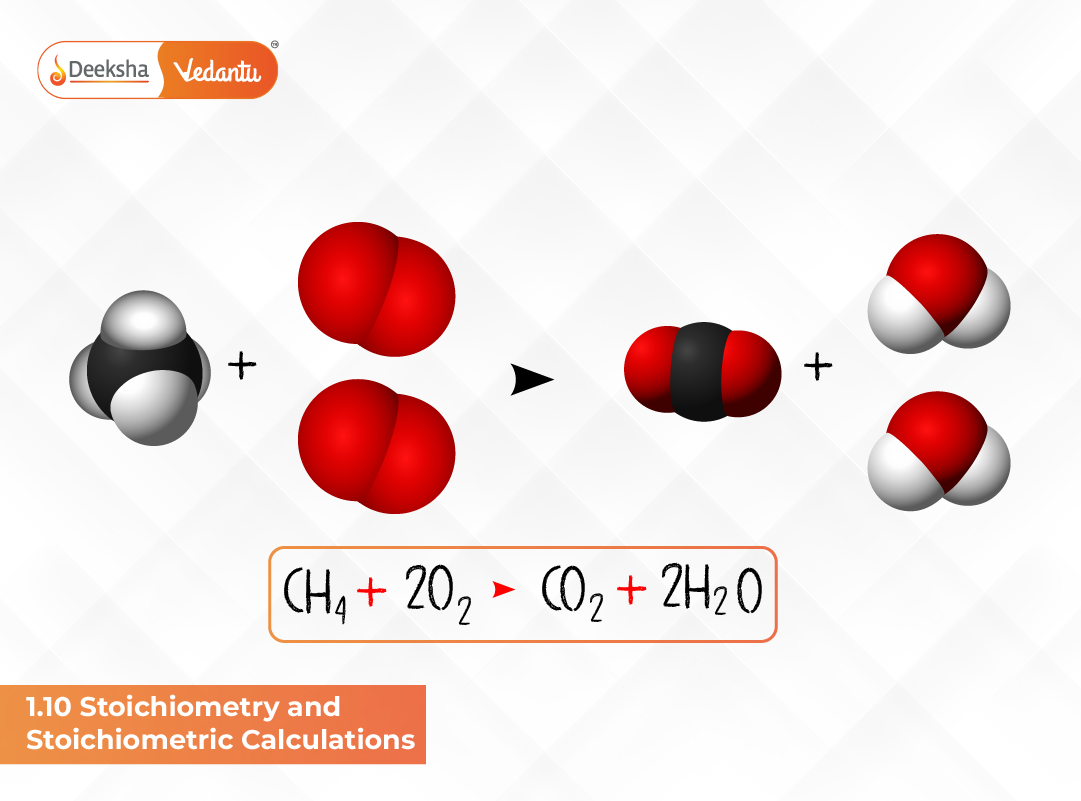
Consider the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
From this balanced equation:
- 1 mole of CH₄ reacts with 2 moles of O₂.
- This produces 1 mole of CO₂ and 2 moles of H₂O.
If we have 16 g of CH₄ (1 mole), it reacts with 64 g of O₂ (2 moles) to produce 44 g of CO₂ and 36 g of H₂O. This simple example highlights how stoichiometric principles are used to determine precise amounts of reactants and products in chemical reactions.
Stoichiometry also extends beyond mere calculation—it forms the backbone of understanding yield efficiency and reaction optimization, both crucial in industrial and academic chemical processes.
Limiting Reagent
In most chemical reactions, reactants are not present in the exact stoichiometric proportions required by the balanced equation. One of the reactants is often completely consumed before the others, and this reactant is known as the limiting reagent (or limiting reactant). Once the limiting reagent is used up, the reaction stops, and no further product can be formed regardless of the amount of other reactants present. The other reactants that remain unreacted are called excess reagents.
Identifying the Limiting Reagent
To determine which reactant is limiting, follow these steps:
- Write and balance the chemical equation.
- Convert all given masses or volumes of reactants to moles.
- Determine the stoichiometric mole ratio between reactants from the balanced equation.
- Compare the available mole ratio with the required stoichiometric ratio.
- The reactant that produces the smallest theoretical amount of product is the limiting reagent.
Example
For the reaction:
2H₂ + O₂ → 2H₂O
If 4 moles of H₂ and 3 moles of O₂ are available:
- The stoichiometric ratio is H₂ : O₂ = 2 : 1.
- For 4 moles of H₂, only 2 moles of O₂ are required.
- Since 3 moles of O₂ are present, O₂ is in excess and H₂ is the limiting reagent.
Thus, hydrogen determines how much water can be produced in this reaction.
Importance of Limiting Reagents
- Limiting reagents are crucial in industrial synthesis to ensure minimal waste and maximum efficiency.
- They help in calculating theoretical yields, which can be compared to actual yields to determine reaction efficiency.
- In practical chemistry, understanding the limiting reagent concept prevents wastage of costly materials and supports process optimization.
Applications of Stoichiometry
- Quantitative Analysis: Used to calculate the exact quantities of reactants or products in a reaction.
- Predicting Reaction Yields: Helps determine theoretical, actual, and percentage yields.
- Industrial Chemistry: Aids in designing cost-effective chemical production processes.
- Environmental Applications: Useful in analyzing pollutant reactions, such as determining the amount of oxygen needed to oxidize carbon monoxide.
- Pharmaceutical Industry: Ensures precise formulation and purity in drug synthesis.
Example
Consider the reaction:
N₂ + 3H₂ → 2NH₃
If 10 g of N₂ and 10 g of H₂ are mixed, find the limiting reagent and the amount of ammonia produced.
Step 1: Convert given masses to moles.
- Moles of N₂ = 10 / 28 = 0.357 mol
- Moles of H₂ = 10 / 2 = 5 mol
Step 2: Compare stoichiometric requirements.
According to the equation, 1 mole of N₂ requires 3 moles of H₂.
Required H₂ = 0.357 × 3 = 1.071 mol
Available H₂ = 5 mol (which is greater than required)
Therefore, N₂ is the limiting reagent.
Step 3: Calculate the amount of NH₃ formed.
- Moles of NH₃ produced = 0.357 × 2 = 0.714 mol
- Mass of NH₃ = 0.714 × 17 = 12.14 g
This example demonstrates how theoretical calculations can accurately predict measurable chemical outcomes, connecting stoichiometry to laboratory and industrial practices.
Key Takeaways
- Stoichiometry is essential for quantifying reactants and products in any chemical reaction.
- A balanced chemical equation is the foundation for accurate calculations.
- The limiting reagent determines how much product can be formed.
- These concepts are crucial for mastering reaction yield problems in JEE and other entrance exams.
- Stoichiometry also plays a vital role in environmental, industrial, and biological chemistry.
FAQs
Q1: What is stoichiometry in simple terms?
Stoichiometry is the study of quantitative relationships between reactants and products in chemical reactions, helping predict how much of each substance is involved.
Q2: Why is stoichiometry important for JEE preparation?
It forms the foundation for solving numerical problems involving reaction yields, limiting reagents, and concentration calculations—concepts that frequently appear in JEE Chemistry.
Q3: What is the limiting reagent and why is it important?
The limiting reagent is the reactant that is completely used up first during a reaction. It limits the amount of product formed and helps determine the theoretical yield.
Q4: Can a reaction have more than one limiting reagent?
Generally, only one reactant limits a reaction at a time. However, in complex multi-step reactions, each step may have a different limiting reagent.
Q5: How is stoichiometry applied in real life?
It is used in industries for accurate chemical production, in laboratories for yield optimization, and even in environmental studies to estimate pollutant reactions and carbon cycles.
Conclusion
Stoichiometry and the concept of the limiting reagent are at the core of chemical problem-solving. By mastering these principles, students not only enhance their analytical skills but also gain the ability to relate theoretical chemistry to real-world applications. Whether it is calculating reaction yields in a laboratory, designing efficient industrial processes, or understanding environmental chemical cycles, stoichiometric analysis provides a reliable framework for quantitative reasoning in chemistry. For JEE aspirants, building a strong command over this topic ensures confidence in solving complex numerical questions and developing a deeper appreciation for chemical reactions.





Get Social