The solubility of ionic solids in water varies dramatically due to differences in lattice energy and solvation enthalpy. These two energetic factors together determine whether a salt dissolves completely, partially, or barely at all. While salts such as calcium chloride are extremely soluble due to their strong solvation enthalpy, others like barium sulfate (BaSO₄) and lithium fluoride (LiF) remain only slightly soluble because of their high lattice enthalpy. These salts are referred to as sparingly soluble salts and play a crucial role in understanding equilibrium in ionic systems.
The extent to which a salt dissolves in a solvent depends on two opposing energy changes:
- Lattice Enthalpy (ΔHlatt): The amount of energy needed to break the ionic lattice and separate the ions in a solid into the gaseous phase.
- Solvation Enthalpy (ΔHsol): The energy released when ions are surrounded and stabilized by solvent molecules, such as water.
For a salt to dissolve spontaneously, the solvation enthalpy must be greater than the lattice enthalpy. If the lattice energy dominates, dissolution is minimal, leading to low solubility. Based on solubility, salts can be classified into the following three categories:
| Category | Description | Solubility Range |
| I | Highly Soluble | > 0.1 M |
| II | Slightly Soluble | 0.01 M – 0.1 M |
| III | Sparingly Soluble | < 0.01 M |
The interplay between these energy factors helps chemists predict whether an ionic compound will remain undissolved, form a saturated solution, or completely dissociate into ions.
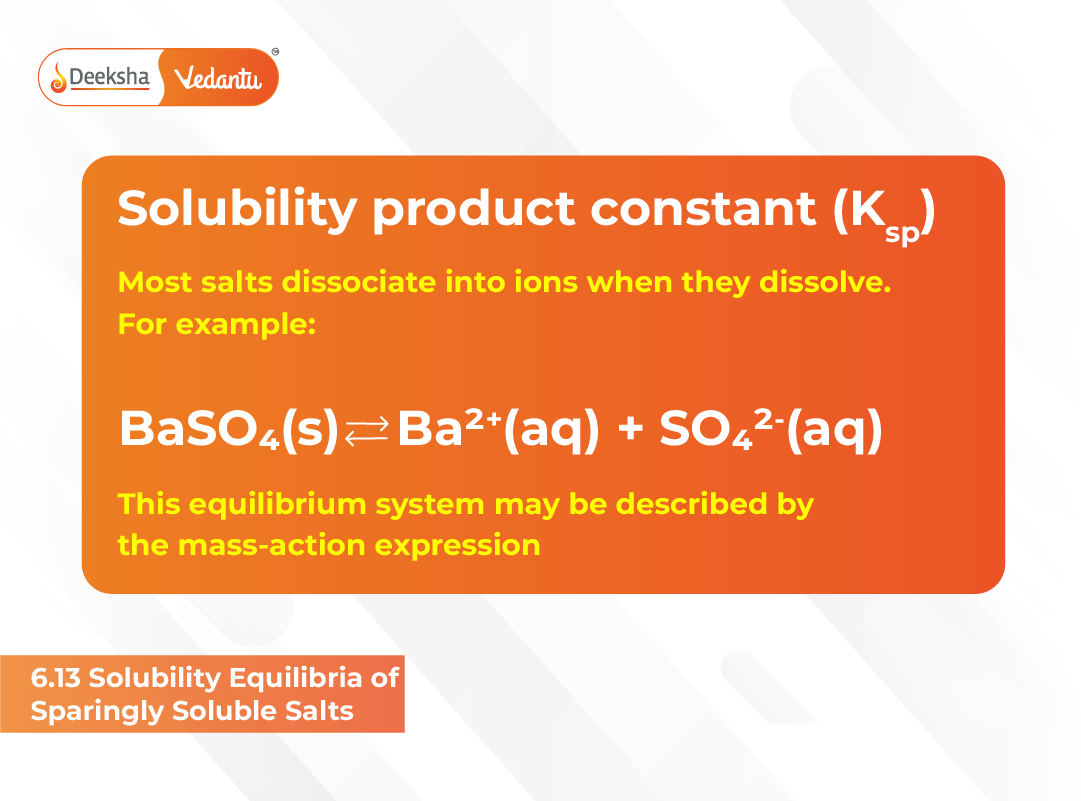
Solubility Product Constant (Ksp)
When a sparingly soluble salt is placed in water, it partially dissolves until equilibrium is established between the solid phase and its dissolved ions. At this stage, the rate of dissolution equals the rate of precipitation, and the system achieves solubility equilibrium.
For example, consider barium sulfate (BaSO₄):
BaSO₄(s) ⇌ Ba²⁺(aq) + SO₄²⁻(aq)
At equilibrium, the product of ionic concentrations is constant for a given temperature:
Ksp = [Ba²⁺][SO₄²⁻]
Here, Ksp, the solubility product constant, is independent of the amount of undissolved solid and depends only on temperature. For BaSO₄, Ksp = 1.1 × 10⁻¹⁰ at 298 K. This means that at equilibrium, the product of Ba²⁺ and SO₄²⁻ concentrations equals 1.1 × 10⁻¹⁰.
If the molar solubility of BaSO₄ is S mol/L, then:
[Ba²⁺] = [SO₄²⁻] = S → Ksp = S² → S = √Ksp = √(1.1 × 10⁻¹⁰) = 1.05 × 10⁻⁵ mol/L.
This indicates that only 1.05 × 10⁻⁵ moles of BaSO₄ dissolve in one liter of water to reach equilibrium.
Solubility Products for Multi-Ionic Salts
For salts that release more than two ions, the equilibrium expression changes according to the stoichiometric coefficients:
- MX₂(s):
MX₂ ⇌ M²⁺ + 2X⁻
Ksp = [M²⁺][X⁻]² = S × (2S)² = 4S³ → S = (Ksp / 4)¹/³ - M₃X₂(s):
M₃X₂ ⇌ 3M²⁺ + 2X³⁻
Ksp = (3S)³(2S)² = 108S⁵ → S = (Ksp / 108)¹/⁵
The smaller the value of Ksp, the less soluble the salt. This property allows chemists to rank salts by solubility and predict precipitation sequences in qualitative analysis.
Example 1: Solubility of A₂X₃
Given Ksp = 1.1 × 10⁻²³ for A₂X₃, where:
A₂X₃ ⇌ 2A³⁺ + 3X²⁻
We have:
Ksp = (2S)²(3S)³ = 108S⁵ → S = (Ksp / 108)¹/⁵
S = (1.1 × 10⁻²³ / 108)¹/⁵ = 1.0 × 10⁻⁵ mol/L.
Thus, only a trace amount of A₂X₃ dissolves at equilibrium.
Example 2: Comparing Solubility of Ni(OH)₂ and AgCN
Given:
Ksp(Ni(OH)₂) = 2 × 10⁻¹⁵, Ksp(AgCN) = 6 × 10⁻¹⁷.
Ni(OH)₂ ⇌ Ni²⁺ + 2OH⁻ → Ksp = [Ni²⁺][OH⁻]² = 4S³
AgCN ⇌ Ag⁺ + CN⁻ → Ksp = S²
Substituting values:
S(Ni(OH)₂) = (2 × 10⁻¹⁵ / 4)¹/³ = 7.8 × 10⁻⁶,
S(AgCN) = √(6 × 10⁻¹⁷) = 7.7 × 10⁻⁹.
Hence, Ni(OH)₂ is significantly more soluble than AgCN.
Common Ion Effect on Solubility
According to Le Chatelier’s Principle, when a common ion is added to a solution, the solubility of the salt decreases. The system adjusts by shifting equilibrium toward the undissolved solid to minimize the disturbance.
For example:
AgCl(s) ⇌ Ag⁺ + Cl⁻
Adding NaCl increases [Cl⁻], forcing the reaction leftward and precipitating more AgCl. This effect is widely observed in salts like PbCl₂, CaF₂, and BaSO₄.
However, in some systems containing basic anions (such as CO₃²⁻ or OH⁻), the addition of acid enhances solubility due to neutralization reactions:
CaCO₃(s) ⇌ Ca²⁺ + CO₃²⁻
CO₃²⁻ + 2H⁺ → CO₂ + H₂O, increasing the overall solubility.
Thus, the solubility of weak-acid salts (carbonates, phosphates, hydroxides) increases significantly in acidic solutions.
Mathematical Expression of pH Dependence
For a salt of a weak acid HX:
Ksp = [M⁺][X⁻], and HX ⇌ H⁺ + X⁻ → Ka = [H⁺][X⁻] / [HX]
Combining these expressions:
S = (Ksp / (1 + [H⁺]/Ka))¹/², showing that solubility increases as [H⁺] increases (or pH decreases). Conversely, solubility decreases in basic solutions.
Experimental Ksp Values (298 K)
| Salt | Formula | Ksp |
| Silver Chloride | AgCl | 1.8 × 10⁻¹⁰ |
| Barium Sulfate | BaSO₄ | 1.1 × 10⁻¹⁰ |
| Calcium Carbonate | CaCO₃ | 8.7 × 10⁻⁹ |
| Lead(II) Sulfate | PbSO₄ | 1.6 × 10⁻⁸ |
| Nickel(II) Hydroxide | Ni(OH)₂ | 2 × 10⁻¹⁵ |
| Silver Cyanide | AgCN | 6 × 10⁻¹⁷ |
These data help in determining solubility order, predicting precipitation, and analyzing ions in laboratory experiments.
Applications of Solubility Product
- Predicting Precipitation: If the ionic product (Q) > Ksp, precipitation occurs; if Q < Ksp, the solution remains unsaturated.
- Determining Solubility: From the Ksp value, solubility can be derived for any salt in pure or mixed solutions.
- Selective Precipitation: Used in qualitative analysis to separate ions based on their solubility differences.
- Purification and Crystallization: Controlled crystallization of sparingly soluble salts allows purification in industry.
- Environmental and Biological Relevance: Explains phenomena like calcium phosphate deposition in bones and carbonate scaling in pipelines.
Conclusion
Understanding solubility equilibria provides insight into how ionic compounds behave in aqueous systems. The concepts of Ksp, Le Chatelier’s principle, and common ion effect enable prediction of solubility, precipitation, and equilibrium adjustments under varying pH and ionic conditions. These principles have far-reaching implications in industrial chemistry, analytical methods, biological processes, and environmental chemistry—making solubility product one of the most significant topics in equilibrium studies.





Get Social