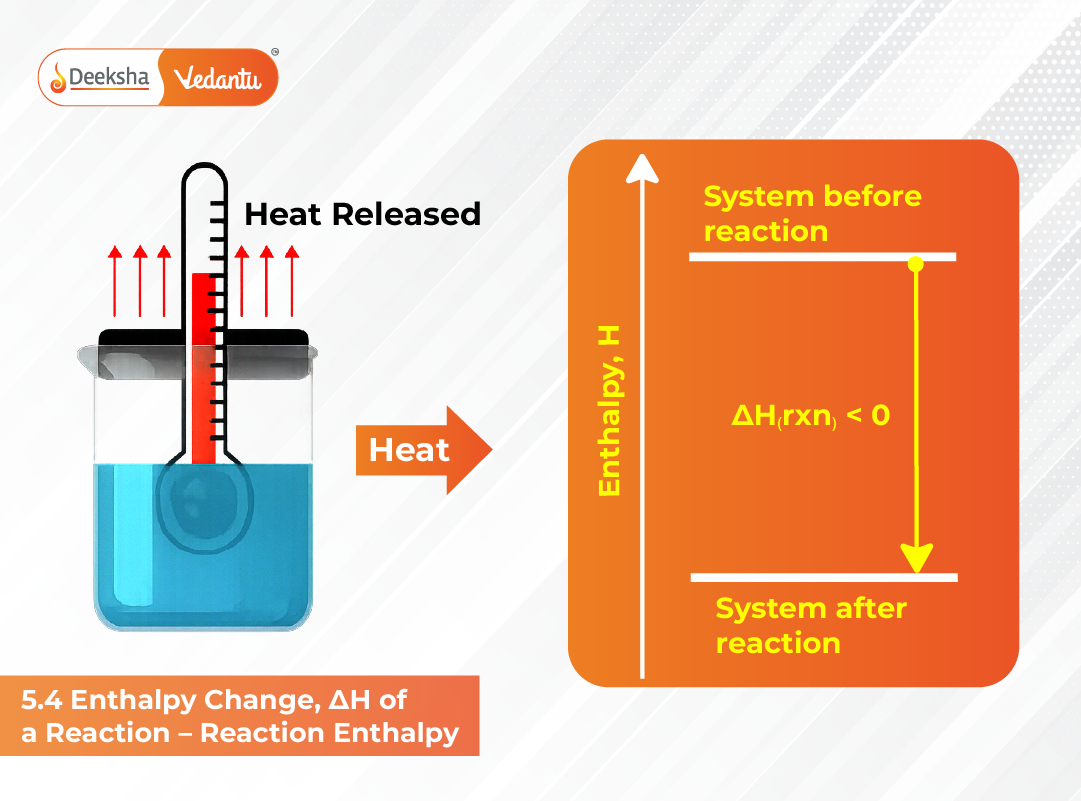
The enthalpy change (ΔH) of a chemical reaction measures the amount of heat absorbed or released when reactants convert into products at constant pressure. It represents the difference between the total enthalpy (heat content) of the products and the reactants. The relationship is expressed as:
ΔH = ΣaH(products) – ΣbH(reactants)
Here, Σa and Σb denote the sum of enthalpies of products and reactants respectively, each multiplied by their stoichiometric coefficients. The value of ΔH indicates the thermal nature of the reaction:
- Negative ΔH (Exothermic Reaction): Heat is released to the surroundings, making products more stable than reactants.
- Positive ΔH (Endothermic Reaction): Heat is absorbed from the surroundings, indicating products have higher energy than reactants.
In essence, enthalpy is a measure of total heat content or energy potential of a substance. It depends on the chemical bonds present in molecules—breaking bonds requires energy, while forming bonds releases energy. The net difference between these two energy changes yields the reaction enthalpy.
Understanding ΔH helps students predict reaction spontaneity, equilibrium behavior, and thermodynamic feasibility — all key topics for competitive exams like NEET and JEE Main/Advanced.
Deep Dive: Reaction Enthalpy and Its Physical Significance
Every chemical reaction is an energy transformation process. Reactants with certain internal energy rearrange their atoms and bonds to form products with different energy content. This change is quantified as reaction enthalpy.
Example:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
ΔH = [H(CO₂) + 2H(H₂O)] – [H(CH₄) + 2H(O₂)]
ΔH = –890.3 kJ mol⁻¹ (exothermic)
This means 890.3 kJ of heat is released per mole of methane combusted under standard conditions. Such information is crucial in fields like energy engineering, environmental chemistry, and combustion analysis.
The magnitude of ΔH also determines whether a reaction is feasible under constant-pressure conditions — a principle frequently used in calorimetric experiments.
Relation Between ΔH and ΔU (Internal Energy)
The First Law of Thermodynamics forms the foundation of this relationship:
ΔH = ΔU + PΔV = ΔU + Δn₍g₎RT
Where:
- ΔU = Change in internal energy
- PΔV = Work done by gas expansion or compression
- Δn₍g₎ = Moles of gaseous products – Moles of gaseous reactants
- R = Gas constant (8.314 J mol⁻¹ K⁻¹)
- T = Temperature in kelvin
When the number of moles of gas does not change (Δn₍g₎ = 0), the enthalpy change equals the internal energy change. This scenario often appears in reactions involving solids and liquids.
Example:
N₂(g) + 3H₂(g) → 2NH₃(g)
Δn₍g₎ = 2 – (1 + 3) = –2
Therefore, ΔH = ΔU – 2RT.
This correction becomes significant in gaseous reactions where pressure-volume work cannot be ignored, such as in combustion, explosions, or gas evolution processes.
Standard Enthalpy of Formation (ΔH°𝒻)
The standard enthalpy of formation (ΔH°𝒻) is defined as the enthalpy change accompanying the formation of one mole of a compound from its constituent elements in their most stable standard states at 298 K and 1 bar pressure.
Examples:
C(graphite) + O₂(g) → CO₂(g); ΔH°𝒻 = –393.5 kJ mol⁻¹
H₂(g) + ½O₂(g) → H₂O(l); ΔH°𝒻 = –285.8 kJ mol⁻¹
Na(s) + ½Cl₂(g) → NaCl(s); ΔH°𝒻 = –411.2 kJ mol⁻¹
Calculation of Reaction Enthalpy using ΔH°𝒻 values:
ΔH°ᵣ = ΣΔH°𝒻(products) – ΣΔH°𝒻(reactants)
Illustration:
CaCO₃(s) → CaO(s) + CO₂(g)
ΔH°ᵣ = [–635.1 + (–393.5)] – (–1206.9) = +178.3 kJ mol⁻¹
This reaction is endothermic — indicating that heat must be supplied to decompose limestone into quicklime and carbon dioxide.
Hess’s Law of Constant Heat Summation – Derivation and Applications
Statement: The total enthalpy change of a chemical reaction is the same, regardless of the route by which the reaction proceeds, provided the initial and final states remain unchanged.
This law highlights that enthalpy is a state function — it depends only on the current state of the system, not the path taken to reach it. Hess’s Law is extensively used to calculate ΔH values for reactions that cannot be measured directly in laboratories.
Mathematical Representation:
If a reaction proceeds in several steps:
ΔH = ΔH₁ + ΔH₂ + ΔH₃ + …
Graphical Illustration:
A ——ΔH₁——> B
| ^
| |
ΔH₃ ΔH₂
| |
v |
C ——ΔH₄——> D
Example Using Hess’s Law:
To calculate ΔH for the reaction:
C(graphite) + ½O₂(g) → CO(g)
Given:
C(graphite) + O₂(g) → CO₂(g); ΔH° = –393.5 kJ mol⁻¹
CO(g) + ½O₂(g) → CO₂(g); ΔH° = –283.0 kJ mol⁻¹
Therefore, ΔH° = (–393.5 + 283.0) = –110.5 kJ mol⁻¹.
This shows that the enthalpy change remains constant whether CO is formed directly or through an indirect two-step route via CO₂.
Hess’s Law helps in determining enthalpies of formation, combustion, neutralization, and transition even when direct calorimetric measurements are impractical.
Thermochemical Equations and Their Importance
A thermochemical equation combines both the quantitative and qualitative aspects of a chemical reaction. It includes physical states and the associated enthalpy change (ΔH) under specified conditions.
Examples:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l); ΔH = –890.3 kJ mol⁻¹ (exothermic)
2NH₃(g) → N₂(g) + 3H₂(g); ΔH = +91.8 kJ mol⁻¹ (endothermic)
Key Rules:
- Always mention the physical states (solid, liquid, gas, or aqueous).
- Reversing a reaction changes the sign of ΔH.
- Multiplying the stoichiometric coefficients by a number multiplies ΔH by the same factor.
- The magnitude of ΔH is specific to the given balanced equation.
Thermochemical equations are used in designing industrial processes (e.g., Haber process, combustion analysis), and for computing the energy efficiency of reactions.
Experimental Measurement – Calorimetry
Calorimetry is the experimental technique used to determine enthalpy changes by measuring heat transfer between a chemical system and its surroundings.
At constant pressure:
q₍p₎ = ΔH = m × s × ΔT
where:
- m = mass of the substance
- s = specific heat capacity
- ΔT = temperature change
Types of Calorimeters:
- Bomb Calorimeter: Used for combustion reactions; operates at constant volume.
- Coffee Cup Calorimeter: Measures heat at constant pressure, ideal for solution reactions.
Example: The combustion of benzoic acid in a bomb calorimeter releases a known amount of heat, used to determine the calorimeter constant and calculate ΔH accurately.
Extended Applications of Enthalpy Concepts
- Enthalpy of Neutralization: Heat released when one mole of water forms from an acid-base reaction.
Example: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l); ΔH = –57.1 kJ mol⁻¹. - Enthalpy of Solution: Heat change when a solute dissolves in a solvent.
- Enthalpy of Fusion/Vaporization: Related to phase changes, measurable from experimental data.
These variations of enthalpy are crucial in biochemical reactions, metallurgy, and engineering thermodynamics.
Summary of Key Learnings
- ΔH = ΣH(products) – ΣH(reactants)
- ΔH = ΔU + Δn₍g₎RT
- Exothermic reactions (ΔH < 0) release heat; Endothermic (ΔH > 0) absorb heat.
- Enthalpy is a state function independent of path.
- Hess’s Law allows indirect ΔH calculations for complex reactions.
- Thermochemical equations must indicate states and sign conventions.
- Calorimetry is a key experimental method to measure ΔH.
FAQs
Q1. What does ΔH signify in chemistry?
It represents the total heat change of a reaction at constant pressure, determining if it’s exothermic or endothermic.
Q2. How is ΔH related to internal energy (ΔU)?
ΔH = ΔU + Δn₍g₎RT, linking enthalpy and energy through gas work.
Q3. Define the standard enthalpy of formation.
It is the heat change when one mole of a compound forms from its elements under standard conditions (298 K, 1 bar).
Q4. State Hess’s Law and its use.
It states that total enthalpy change depends only on initial and final states. It’s used to calculate ΔH for reactions that can’t be measured directly.
Q5. Why are thermochemical equations essential?
They represent both chemical and energetic transformations, providing quantitative energy data crucial for industrial and laboratory processes.
Q6. What instruments measure enthalpy changes experimentally?
Calorimeters like the bomb and coffee-cup calorimeter are used to determine heat flow and calculate enthalpy precisely.
Conclusion
The concept of enthalpy change (ΔH) bridges theory and practical chemistry. It allows prediction of reaction behavior, energy efficiency, and heat management in both natural and industrial processes. Using NCERT principles, Hess’s Law, and standard enthalpy data, students can calculate complex heat changes accurately — a skill invaluable for mastering NEET and JEE Chemistry.





Get Social